Progestogen for preventing miscarriage in women with recurrent miscarriage of unclear etiology
- PMID: 31745982
- PMCID: PMC6953238
- DOI: 10.1002/14651858.CD003511.pub5
Progestogen for preventing miscarriage in women with recurrent miscarriage of unclear etiology
Update in
-
Progestogen for preventing miscarriage in women with recurrent miscarriage of unclear etiology.Cochrane Database Syst Rev. 2025 Jun 11;6(6):CD003511. doi: 10.1002/14651858.CD003511.pub6. Cochrane Database Syst Rev. 2025. PMID: 40497447
Abstract
Background: Progesterone, a female sex hormone, is known to induce secretory changes in the lining of the uterus essential for successful implantation of a fertilized egg. It has been suggested that a causative factor in many cases of miscarriage may be inadequate secretion of progesterone. Therefore, clinicians use progestogens (drugs that interact with the progesterone receptors), beginning in the first trimester of pregnancy, in an attempt to prevent spontaneous miscarriage. This is an update of a review, last published in 2013. Since publication of the 2018 update of this review, we have been advised that the Ismail 2017 study is currently the subject of an investigation by the Journal of Maternal-Fetal & Neonatal Medicine. We have now moved this study from 'included studies' to 'Characteristics of studies awaiting classification' until the outcome of the investigation is known.
Objectives: To assess the efficacy and safety of progestogens as a preventative therapy against recurrent miscarriage.
Search methods: For this update, we searched Cochrane Pregnancy and Childbirth's Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (6 July 2017) and reference lists from relevant articles, attempting to contact trial authors where necessary, and contacted experts in the field for unpublished works.
Selection criteria: Randomized or quasi-randomized controlled trials comparing progestogens with placebo or no treatment given in an effort to prevent miscarriage.
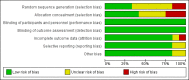
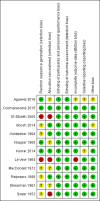
Data collection and analysis: Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy. Two reviewers assessed the quality of the evidence using the GRADE approach.
Main results: Twelve trials (1,856 women) met the inclusion criteria. Eight of the included trials compared treatment with placebo and the remaining four trials compared progestogen administration with no treatment. The trials were a mix of multicenter and single-center trials, conducted in India, Jordan, UK and USA. In five trials women had had three or more consecutive miscarriages and in seven trials women had suffered two or more consecutive miscarriages. Routes, dosage and duration of progestogen treatment varied across the trials. The majority of trials were at low risk of bias for most domains. Ten trials (1684 women) contributed data to the analyses. The meta-analysis of all women, suggests that there may be a reduction in the number of miscarriages for women given progestogen supplementation compared to placebo/controls (average risk ratio (RR) 0.73, 95% confidence interval (CI) 0.54 to 1.00, 10 trials, 1684 women, moderate-quality evidence). A subgroup analysis comparing placebo-controlled versus non-placebo-controlled trials, trials of women with three or more prior miscarriages compared to women with two or more miscarriages and different routes of administration showed no clear differences between subgroups for miscarriage. None of the trials reported on any secondary maternal outcomes, including severity of morning sickness, thromboembolic events, depression, admission to a special care unit, or subsequent fertility. There was probably a slight benefit for women receiving progestogen seen in the outcome of live birth rate (RR 1.07, 95% CI 1.00 to 1.13, 6 trials, 1411 women, moderate-quality evidence). We are uncertain about the effect on the rate of preterm birth because the evidence is very low-quality (RR 1.13, 95% CI 0.53 to 2.41, 4 trials, 256 women, very low-quality evidence). No clear differences were seen for women receiving progestogen for the other secondary outcomes including neonatal death, fetal genital abnormalities or stillbirth. There may be little or no difference in the rate of low birthweight and trials did not report on the secondary child outcomes of teratogenic effects or admission to a special care unit.
Authors' conclusions: For women with unexplained recurrent miscarriages, supplementation with progestogen therapy may reduce the rate of miscarriage in subsequent pregnancies.
Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
David M Haas: none known
Taylor J Hathaway: none known
Patrick S Ramsey: I serve as an officer in Section 5 of District XI of the American College and Congress of Obstetricians and Gynecologists. I received compensation for this general work with ACOG but have not engaged in any specific work relevant to the topic of this review. I also serve on the Data Safety Monitoring Board for the KV Pharmaceutical RCT designed to evaluate the efficacy of 17‐hydroxyprogesterone caproate to prevent preterm birth. I received compensation for service as the DSMB chair from an independent data management organization and not from the pharmaceutical company.
Figures
Update of
-
Progestogen for preventing miscarriage in women with recurrent miscarriage of unclear etiology.Cochrane Database Syst Rev. 2018 Oct 8;10(10):CD003511. doi: 10.1002/14651858.CD003511.pub4. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2019 Nov 20;2019(11). doi: 10.1002/14651858.CD003511.pub5. PMID: 30298541 Free PMC article. Updated.
References
References to studies included in this review
Agarwal 2016 {published data only}
-
- CTRI/2016/09/007278. Role of inflammatory markers in recurrent pregnancy loss and effect of oral micronized therapy on these cases. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=14740 (first received 16 September 2016).
Coomarasamy 2015 {published data only}
-
- Coomarasamy A, Williams H, Truchanowicz E, Seed PT, Small R, Quenby S, et al. A randomized trial of progesterone in women with recurrent miscarriages. New England Journal of Medicine 2015;373(22):2141‐8. - PubMed
-
- Coomarasamy A, Williams H, Truchanowicz E, Seed PT, Small R, Quenby S, et al. PROMISE: first‐trimester progesterone therapy in women with a history of unexplained recurrent miscarriages ‐ a randomised, double‐blind, placebo‐controlled, international multicentre trial and economic evaluation. Health Technology Assessment 2016;20(41):7‐91. - PMC - PubMed
-
- ISRCTN92644181. First trimester progesterone therapy in women with a history of unexplained recurrent miscarriages: a randomised double‐blind placebo‐controlled multi‐centre trial (The PROMISE [PROgesterone in recurrent MIScarriagE] Trial). controlled‐trials.com/ISRCTN92644181 (first received 17 March 2009). - PMC - PubMed
El‐Zibdeh 2005 {published data only}
-
- Zibdeh M. Randomized clinical trial comparing the efficacy of dydrogesterone and human chorionic gonadotropin. Climacteric 2002;5(Suppl 1):136.
-
- Zibdeh M. Randomized study comparing the efficacy of reducing spontaneous abortion following treatment with progesterone and human chorionic gonadotropin (hCG). Fertility and Sterility 1998;70(3 Suppl 1):S77‐S78.
-
- El‐Zibdeh MY. Dydrogesterone in the reduction of recurrent spontaneous abortion. Journal of Steroid Biochemistry & Molecular Biology 2005;97(5):431‐4. - PubMed
Ghosh 2014 {published data only}
-
- CTRI/2013/02/003406. Assessment of sub‐endometrial blood flow parameters following dydrogesterone and micronized vaginal progesterone administration in women with idiopathic recurrent spontaneous miscarriage. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=5847 (first received 18 February 2013).
-
- Chakravarty BN, Ganesh A, Chowdhuri K, Shyam T, Ghosh S, Chattopadhyay R. Assessment of endometrial vascularity following dydrogesterone and micronized progesterone administration in idiopathic recurrent miscarriage‐a preliminary study. Human Reproduction 2012;27(Suppl 2):P089.
-
- Ghosh S, Chattopadhyay R, Goswami S, Chaudhury K, Chakravarty B, Ganesh A. Assessment of sub‐endometrial blood flow parameters following dydrogesterone and micronized vaginal progesterone administration in women with idiopathic recurrent miscarriage: a pilot study. Journal of Obstetrics & Gynaecology Research 2014;40(7):1871‐6. - PubMed
Goldzieher 1964 {published data only}
-
- Goldzieher JW. Double‐blind trial of a progestin in habitual abortion. JAMA 1964;188(7):651‐4. - PubMed
Klopper 1965 {published data only}
-
- Klopper A, MacNaughton M. Hormones in recurrent abortion. Journal of Obstetrics and Gynaecology of the British Commonwealth 1965;72:1022‐8.
Kumar 2014 {published data only}
-
- Kumar A, Begum N, Prasad S, Aggarwal S, Sharma S. Oral dydrogesterone treatment during early pregnancy to prevent recurrent pregnancy loss and its role in modulation of cytokine production: a double‐blind, randomized, parallel, placebo‐controlled trial. Fertility and Sterility 2014;102(5):1357‐63.e3. - PubMed
Le Vine 1964 {published data only}
-
- Vine L. Habitual abortion. A controlled clinical study of progestational therapy. Western Journal of Surgery 1964;72:30‐6. - PubMed
MacDonald 1972 {published data only}
-
- MacDonald RR, Goulden R, Oakey RE. Cervical mucus, vaginal cytology and steroid excretion in recurrent abortion. Obstetrics & Gynecology 1972;40(3):394‐402. - PubMed
Reijnders 1988 {published data only}
-
- Reijnders FJ, Thomas CM, Doesburg WH, Rolland R, Eskes TK. Endocrine effects of 17 alpha‐hydroxyprogesterone caproate during early pregnancy: a double‐blind clinical trial. British Journal of Obstetrics and Gynaecology 1988;95:462‐8. - PubMed
Shearman 1963 {published data only}
-
- Shearman RP. Hormonal treatment of habitual abortion. In: UK editor(s). In: Progress In Infertility. Kistner RW (ed). Shearman 1963. London: J & A Churchill, 1968:767‐77.
References to studies excluded from this review
Berle 1980 {published data only}
-
- Berle P, Budenz M, Michaelis J. Is hormonal therapy still justified in imminent abortion?. Zeitschrift fur Geburtshilfe und Perinatologie 1980;184:353‐8. - PubMed
Brenner 1962 {published data only}
-
- Brenner W, Hendricks C. Effect of medroxyprogesterone acetate upon the duration and characteristics of human gestation labour. American Journal of Obstetrics and Gynecology 1962;83(8):1094‐8. - PubMed
Check 1985 {published data only}
-
- Check J, Wu C‐H, Adelson H. Decreased abortion in HMG‐induced pregnancies. International Journal of Fertility 1985;30(3):45‐7. - PubMed
Check 1987a {published data only}
-
- Check J, Chase J, Nowroozi K, Wu C, Adelson H. Progesterone therapy to decrease first‐trimester spontaneous abortions in previous aborters. International Journal of Fertility 1987;32(3):192‐9. - PubMed
Check 1987b {published data only}
-
- Check J, Chase J, Wu C, Adelson H, Teichman M, Rankin A. The efficacy of progesterone in achieving successful pregnancy: prophylactic use during luteal phase in anovulatory women. International Journal of Fertility 1987;32(2):135‐8. - PubMed
Check 1995 {published data only}
-
- Check JH, Tarquini P, Gandy P, Lauer C. A randomized study comparing the efficacy of reducing the spontaneous abortion rate following lymphocyte immunotherapy and progesterone treatment vs progesterone alone in primary habitual aborters. Gynecologic and Obstetric Investigation 1995;39:257‐61. - PubMed
Clifford 1996 {published data only}
Corrado 2002 {published data only}
-
- Corrado F, Dugo C, Cannata M, Bartolo M, Scilipoti A, Stella N. A randomised trial of progesterone prophylaxis after midtrimester amniocentesis. European Journal of Obstetrics & Gynecology and Reproductive Biology 2002;100(2):196‐8. - PubMed
Daya 1988 {published data only}
-
- Daya S, Ward S, Burrows E. Progesterone profiles in luteal phase defect cycles and outcome of progesteron treatment in patients with recurrent spontaneous abortion. American Journal of Obstetrics and Gynecology 1988;158(2):225‐32. - PubMed
Fuchs 1966 {published data only}
-
- Fuchs F, Olsen P. An attempted double‐blind controlled trial of progesterone therapy in habitual abortion. Ugeskrift for Laeger 1966;128:1461‐2. - PubMed
Gerhard 1987 {published data only}
-
- Gerhard I, Gwinner B, Eggert‐Kruse W, Runnebaum B. Double‐blind controlled trial of progesterone substitution in threatened abortion. Biological Research in Pregnancy and Perinatology 1987;8:26‐34. - PubMed
Johnson 1975 {published data only}
-
- Johnson J, Austin K, Jones G, Davis G, King T. Efficacy of 17 alpha‐hydroxyprogesterone caproate on the prevention of premature labour. New England Journal of Medicine 1975;298(14):675‐80. - PubMed
Kyrou 2011 {published data only}
-
- Kyrou D, Fatemi HM, Zepiridis L, Riva A, Papanikolaou EG, Tarlatzis BC, et al. Does cessation of progesterone supplementation during early pregnancy in patients treated with recFSH/GnRH antagonist affect ongoing pregnancy rates? A randomized controlled trial. Human Reproduction 2011;26(5):1020‐4. - PubMed
Moller 1965 {published data only}
-
- Moller K, Fuchs F. Double blind controlled trial of 6‐methyl‐17‐acetoxyprogesterone in threatened abortion. Journal of Obstetrics and Gynaecology of the British Commonwealth 1965;72:1042‐4.
Norman 2006 {published data only}
-
- Norman JE. Double blind randomised placebo controlled study of progesterone for the prevention of preterm birth in twins (STOPPIT). National Research Register. www.nrr.nhs.uk (accessed 6 July 2006).
Nyboe Anderson 2002 {published data only}
-
- Nyboe Anderson A, Popovic‐Todorovic B, Schmidt K, Loft A, Lindhard A, Hojgaard A, et al. Progesterone supplement during early gestations after IVF or ICSI has no effect on the delivery rates: a randomized controlled trial. Human Reproduction 2002;17(2):357‐61. - PubMed
Prietl 1992 {published data only}
-
- Prietl G, Diedrich K, Ven HH, Luckhaus J, Krebs D. The effect of 17alpha‐hydroxyprogesterone caproate/oestradiol valerate on the development and outcome of early pregnancies following in vitro fertilization and embryo transfer: a prospective and randomized controlled trial. Human Reproduction 1992;7(1):1‐5. - PubMed
Rock 1985 {published data only}
-
- Rock J, Colston Wentz A, Cole K, Kimball A, Zacur H, Early S, et al. Fetal malformations following progesterone therapy during pregnancy: a preliminary report. Fertility and Sterility 1985;44(1):17‐9. - PubMed
Shu 2002 {published data only}
-
- Shu J, Miao P, Wang RJ. Clinical observation on effect of Chinese herbal medicine plus human chorionic gonadotropin and progesterone in treating anticardiolipin antibody‐positive early recurrent spontaneous abortion. Zhongguo Zhong xi yi jie he za zhi Zhongguo Zhongxiyi jiehe zazhi//Chinese Journal of Integrated Traditional and Western Medicine 2002;22(6):414‐6. - PubMed
Sidelnikova 1990 {published data only}
-
- Sidelnikova VM, Demidova EM, Borisova IF, Dondukova TM, Absava GI, Korkhov VV. The use of acetomepegrenol in the therapy of threatened abortion. Akusherstvo i Ginekologiia 1990;66(9):37‐40. - PubMed
Smitz 1992 {published data only}
-
- Smitz J, Devroey P, Faguer B, Bourgain C, Camus M, Steirteghem AC. Randomized prospective trial comparing supplementation of the luteal phase and of early pregnancy by natural progesterone given by intramuscular or vaginal administration. Revue Francaise de Gynecologie et d Obstetrique 1992;87:507‐16. - PubMed
Sondergaard 1985 {published data only}
-
- Sondergaard F, Ottesen B, Detlefsen GU, Schierup L, Pederson SC, Lebech PE. Progesterone treatment of cases of threatened pre‐term delivery in women with a low level of plasma progesterone. Contraception, Fertilite, Sexualite 1985;13(12):1227‐31.
Tognoni 1980 {published data only}
-
- Tognoni G, Ferrario L, Inzalaco M, Crosignani P. Progestogens in threatened abortion. Lancet 1980;2(8206):1242‐3. - PubMed
Turner 1966 {published data only}
-
- Turner SJ, Mizock GB, Feldman GL. Prolonged gynecologic and endocrine manifestations subsequent to administration of medroxyprogesterone acetate during pregnancy. American Journal of Obstetrics and Gynecology 1966;95:222‐7. - PubMed
References to studies awaiting assessment
Ismail 2017 {published data only}
-
- Ismail A, Nasr A, Amin A, Al‐Inany H. Peri‐conceptional progesterone treatment in women with unexplained recurrent miscarriage, a randomized double‐blind controlled trial. Human Reproduction 2015;30:i194, abstract no: P‐167.
-
- Ismail AM, Abbas AM, Ali MK, Amin AF. Peri‐conceptional progesterone treatment in women with unexplained recurrent miscarriage: a randomized double‐blind placebo‐controlled trial. Journal of Maternal‐Fetal & Neonatal Medicine 23 January 2017 [Epub ahead of print]. - PubMed
-
- NCT01670929. Pr‐conceptional progesterone for unexplained recurrent miscarriage. clinicaltrials.gov/ct2/show/record/NCT01670929 (first received 17 August 2012).
References to ongoing studies
ACTRN12611000401954 {published data only}
-
- ACTRN12611000401954. Does using progesterone reduce the miscarriage rate in high risk pregnancies? Pregnancy Maintenance Trial (PMTrial). www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12611000401954 18 April 2011.
IRCT2013100114853N1 {published data only}
-
- IRCT2013100114853N1. Effect of concomitant administration of vaginal progesterone and vitamin D3 in the treatment of unexplained recurrent abortion in pregnant women. en.search.irct.ir/view/15255 (first received 30 October 2013).
NCT00193674 {published data only}
-
- NCT00193674. Oral dydrogesterone treatment during the first trimester of pregnancy in women with recurrent miscarriage: a double‐blind, prospectively randomized, placebo‐controlled, parallel group trial. clinicaltrials.gov/ct2/show/NCT00193674 (first received 11 Septamber 2005).
NCT02706470 {published data only}
-
- NCT02706470. A randomized, controlled trial of cyclosporin a for women with unexplained recurrent miscarriage. clinicaltrials.gov/ct2/show/record/NCT02706470 (first received 12 March 2016).
Walch 2005 {published data only}
-
- Walch K, Hefler L, Nagele F. Oral dydrogesterone treatment during the first trimester of pregnancy: the prevention of miscarriage study (PROMIS). A double‐blind, prospectively randomized, placebo‐controlled, parallel group trial. Journal of Maternal‐Fetal & Neonatal Medicine 2005;18(4):265‐9. - PubMed
Additional references
Alfirevic 2012
Atkin 1998
-
- Atkin LC, Arcelus M, Fernandez A, Tolbert K. La Psicologia en el Ambito Perinatal. Mexico D.F: INPER, 1988.
Bamigboye 2003
Briggs 2011
-
- Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation. 9th Edition. Philadelphia: Lippincott Williams & Wilkins, 2011.
Burgoyne 1991
-
- Burgoyne PS, Holland K, Stephens R. Incidence of numerical chromosome anomalies in human pregnancy estimation from induced and spontaneous abortion data. Human Reproduction 1991;6(4):555‐65. - PubMed
Coomarasamy 2011
-
- Coomarasamy A, Truchanowicz EG, Rai R. Does first trimester progesterone prophylaxis increase the live birth rate in women with unexplained recurrent miscarriages?. BMJ 2011;342:d1914. - PubMed
Coulam 1991
-
- Coulam CB. Epidemiology of recurrent spontaneous abortion. American Journal of Reproductive Immunology 1991;26:23‐7. - PubMed
Deeks 2017
-
- Deeks JJ, Higgins JP, Altman DG (editors) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Dodd 2013
Dyregrove 1987
-
- Dyregrove A, Mathieson SB. Stillbirth, neonatal death and SIDS: parental reactions. Scandanavian Journal of Psychology 1987;28:104‐14. - PubMed
Goldstein 1989
-
- Goldstein P, Berrier J, Rosen S, Sacks HS, Chalmers TC. A meta‐analysis of randomised control trials of progestational agents in pregnancy. British Journal of Obstetrics and Gynaecology 1989;96:265‐74. - PubMed
GRADEpro GDT 2015 [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed August 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Grudzinskas 1995
-
- Grudzinskas JG. Endocrinocolgical and metabolical assessment of early pregnancy. In: Chamberlain G editor(s). Tumbull's Obstetrics. London: Pearson Professional Ltd, 1995.
Higgins 2003
Higgins 2011
-
- Higgins JP, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Higgins 2017
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available fromwww.training.cochrane.org/handbook.
Howie 1995
-
- Howie PG. Abortion and ectopic pregnancy. In: Whitfield CR editor(s). Dewhurst's Textbook of Obstetrics and Gynaecology for Postgraduates. Oxford: Blackwell Science Ltd, 1995.
Karamadian 1992
-
- Karamadian LM, Grimes DA. Luteal phase deficiency. Effect of treatment on pregnancy rates. American Journal of Obstetrics and Gynecology 1992;167:1391‐8. - PubMed
Keirse 1990
-
- Keirse MJ. Progestogen administration in pregnancy may prevent preterm delivery. British Journal of Obstetrics and Gynaecology 1990;97(2):149‐54. - PubMed
Lede 2005
McBride 1991
-
- McBride WZ. Spontaneous abortion. American Family Physician 1991;43:175‐82. - PubMed
Morley 2013
Neilson 2006
Porter 2006
Regan 1989
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Royal 2001
-
- Royal College of Obstetricians and Gynaecologists. The management of recurrent miscarriage. www.rcog.org.uk/guidelines/recurrent.html (accessed 2001).
Rumbold 2011
Saccone 2017
-
- Saccone G, Schoen C, Franasiak JM, Scott RT Jr, Berghella V. Supplementation with progestogens in the first trimester of pregnancy to prevent miscarriage in women with unexplained recurrent miscarriage: a systematic review and meta‐analysis of randomized, controlled trials. Fertility and Sterility 2017;107(2):430‐8. - PubMed
Schulz 2010
-
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Annals of Internal Medicine 2010;152:Epub 24 March. - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A (editors). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Updated October 2013. Available from gdt.gradepro.org/app/handbook/handbook.html.
Simpson 1991
-
- Simpson JL. Fetal wastage. In: Gabe SG, Simpson JL editor(s). Obstetrics. Normal and Problem Pregnancies. New York: Churchill Livingstone Inc, 1991.
Sterne 2017
-
- Sterne JA, Egger M, Moher D, Boutron I (editors). Chapter10: Addressing reporting biases. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Szabo 1996
-
- Szabo I, Szilagyi A. Management of threatened abortion. Early Pregnancy 1996;2(4):233‐40. - PubMed
Vance 1991
-
- Vance JC, Foster WJ, Najman JM, Embelton, Thearle MJ, Hogden FM. Early parental responses to sudden infant death, stillbirth or neonatal death. Medical Journal of Australia 1991;155(5):292‐7. - PubMed
Wahabi 2018
Woods 1987
-
- Woods JR, Esposito JL. Pregnancy Loss: Medical Therapeutics and Practical Considerations. Baltimore: Williams and Wilkins, 1987.
References to other published versions of this review
Haas 2008
Haas 2013
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

