LncRNA SPRY4‑IT1 promotes progression of osteosarcoma by regulating ZEB1 and ZEB2 expression through sponging of miR‑101 activity
- PMID: 31746422
- PMCID: PMC6910200
- DOI: 10.3892/ijo.2019.4910
LncRNA SPRY4‑IT1 promotes progression of osteosarcoma by regulating ZEB1 and ZEB2 expression through sponging of miR‑101 activity
Abstract
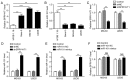
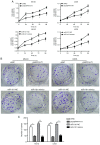
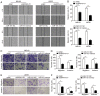
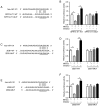
Long non‑coding (lnc)RNA sprouty receptor tyrosine kinase signalling antagonist 4‑intronic transcript 1 (SPRY4‑IT1) has been demonstrated to serve a critical role in the tumorigenesis of osteosarcoma (OS); however, the specific underlying mechanism remains unclear. The aim of the present study was to examine the interactions between SPRY4‑IT1 and its downstream effectors, to determine if any of the interactions contributed to SPRY4‑IT1‑mediated proliferation, migration and invasion in cancer cells. A signalling cascade which involved SPRY4‑IT1, miR‑101 and zinc finger E‑box‑binding homeoboxes (ZEBs) was examined in the present study. Intracellular SPRY4‑IT1 and miR‑101 expression levels were altered through transfection to assess their effect on proliferation, cell cycle progression, survival, migration and invasion. A dual‑luciferase assay was utilized to determine the association between SPRY4‑IT1/miR‑101 and ZEBs/miR‑101 and nude mouse xenograft experiments were performed to determine the effect of SPRY4‑IT1 in vivo. The results indicated that the SPRY4‑IT1 levels were negatively associated with miR‑101 expression levels in OS cells, an association which was not observed in the normal osteoblast cells. SPRY4‑IT1 knockdown or miR‑101 overexpression reduced proliferation, cell cycle progression, survival, migration and invasion of MG‑63 and U2OS cells. SPRY4‑IT1 knockdown was accompanied by increased expression of miR‑101 and E‑cadherin levels, as well as decreased expression levels of ZEB1/2 and other epithelial‑mesenchymal transition‑associated proteins. Simultaneous knockdown of SPRY4‑IT1 and inhibition of miR‑101 partially reversed the anti‑tumour effects of SPRY4‑IT1 inhibition in vitro. Consistent with these findings, short hairpin RNA targeting SPRY4‑IT1 also hindered xenograft tumour growth and altered the levels of miR‑101, ZEB1/2 and E‑cadherin in vivo. Dual‑luciferase reporter assays demonstrated that SPRY4‑IT1 may have regulated the expression of ZEB1 and ZEB2 by sponging miR‑101. In conclusion, SPRY4‑IT1 inhibition increased miR‑101 levels, resulting in downregulation of ZEB1/2 expression and thus exerting anti‑tumour effects in OS.
Figures







Similar articles
-
Oncogenic functions of ZEB1 in pediatric solid cancers: interplays with microRNAs and long noncoding RNAs.Mol Cell Biochem. 2021 Nov;476(11):4107-4116. doi: 10.1007/s11010-021-04226-x. Epub 2021 Jul 22. Mol Cell Biochem. 2021. PMID: 34292482 Review.
-
The microRNA‑708‑5p/ZEB1/EMT axis mediates the metastatic potential of osteosarcoma.Oncol Rep. 2020 Feb;43(2):491-502. doi: 10.3892/or.2019.7452. Epub 2019 Dec 31. Oncol Rep. 2020. PMID: 31894343 Free PMC article.
-
The role and clinical significance of long noncoding RNA zinc finger E-box-binding homeobox two antisense RNA 1 in promoting osteosarcoma cancer cell proliferation, inhibiting apoptosis and increasing migration by regulating miR-145.Anticancer Drugs. 2021 Feb 1;32(2):168-177. doi: 10.1097/CAD.0000000000000984. Anticancer Drugs. 2021. PMID: 32826416
-
lncRNA nuclear-enriched abundant transcript 1 promotes cell proliferation and invasion by targeting miR-186-5p/HIF-1α in osteosarcoma.J Cell Biochem. 2019 Apr;120(4):6502-6514. doi: 10.1002/jcb.27941. Epub 2018 Nov 28. J Cell Biochem. 2019. PMID: 30485482
-
Dualistic role of ZEB1 and ZEB2 in tumor progression.Biol Direct. 2025 Mar 20;20(1):32. doi: 10.1186/s13062-025-00604-3. Biol Direct. 2025. PMID: 40114235 Free PMC article. Review.
Cited by
-
LncRNAs as potential prognosis/diagnosis markers and factors driving drug resistance of osteosarcoma, a review.Front Endocrinol (Lausanne). 2024 Jul 2;15:1415722. doi: 10.3389/fendo.2024.1415722. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39015175 Free PMC article. Review.
-
Long non-coding RNA FGD5-AS1 enhances osteosarcoma cell proliferation and migration by targeting miR-506-3p/RAB3D axis.Hum Cell. 2021 Jul;34(4):1255-1265. doi: 10.1007/s13577-021-00536-w. Epub 2021 Apr 23. Hum Cell. 2021. PMID: 33891267
-
Identification of Differentially Expressed Intronic Transcripts in Osteosarcoma.Noncoding RNA. 2022 Oct 25;8(6):73. doi: 10.3390/ncrna8060073. Noncoding RNA. 2022. PMID: 36412907 Free PMC article.
-
EZH2: A Crucial Competing Endogenous RNA in Cancer Research-A Scoping Review.Adv Biomed Res. 2025 May 31;14:53. doi: 10.4103/abr.abr_561_24. eCollection 2025. Adv Biomed Res. 2025. PMID: 40519579 Free PMC article. Review.
-
Oncogenic functions of ZEB1 in pediatric solid cancers: interplays with microRNAs and long noncoding RNAs.Mol Cell Biochem. 2021 Nov;476(11):4107-4116. doi: 10.1007/s11010-021-04226-x. Epub 2021 Jul 22. Mol Cell Biochem. 2021. PMID: 34292482 Review.
References
-
- Zhang X, Lei X. Expression of Zeb1 and Zeb2 indicates metastasis and unfavorable prognosis in osteosarcoma. Int J Clin Exp Pathol. 2017;10:611–617.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

