DNAzyme-Mediated Genetically Encoded Sensors for Ratiometric Imaging of Metal Ions in Living Cells
- PMID: 31746514
- PMCID: PMC6982602
- DOI: 10.1002/anie.201912514
DNAzyme-Mediated Genetically Encoded Sensors for Ratiometric Imaging of Metal Ions in Living Cells
Abstract
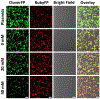
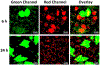
Genetically encoded fluorescent proteins (FPs) have been used for metal ion detection. However, their applications are restricted to a limited number of metal ions owing to the lack of available metal-binding proteins or peptides that can be fused to FPs and the difficulty in transforming the binding of metal ions into a change of fluorescent signal. We report herein the use of Mg2+ -specific 10-23 or Zn2+ -specific 8-17 RNA-cleaving DNAzymes to regulate the expression of FPs as a new class of ratiometric fluorescent sensors for metal ions. Specifically, we demonstrate the use of DNAzymes to suppress the expression of Clover2, a variant of the green FP (GFP), by cleaving the mRNA of Clover2, while the expression of Ruby2, a mutant of the red FP (RFP), is not affected. The Mg2+ or Zn2+ in HeLa cells can be detected using both confocal imaging and flow cytometry. Since a wide variety of metal-specific DNAzymes can be obtained, this method can likely be applied to imaging many other metal ions, expanding the range of the current genetically encoded fluorescent protein-based sensors.
Keywords: DNAzymes; fluorescent proteins; genetically encoded sensors; metal ions.
© 2020 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



Similar articles
-
DNAzyme-Mediated Genetically Encoded Sensors for Ratiometric Imaging of Metal Ions in Living Cells.Angew Chem Weinheim Bergstr Ger. 2020 Jan 27;132(5):1907-1912. doi: 10.1002/ange.201912514. Epub 2019 Nov 20. Angew Chem Weinheim Bergstr Ger. 2020. PMID: 36312441 Free PMC article.
-
DNAzymes as Activity-Based Sensors for Metal Ions: Recent Applications, Demonstrated Advantages, Current Challenges, and Future Directions.Acc Chem Res. 2019 Dec 17;52(12):3275-3286. doi: 10.1021/acs.accounts.9b00419. Epub 2019 Nov 13. Acc Chem Res. 2019. PMID: 31721559 Free PMC article. Review.
-
Metal-Dependent DNAzymes for the Quantitative Detection of Metal Ions in Living Cells: Recent Progress, Current Challenges, and Latest Results on FRET Ratiometric Sensors.Inorg Chem. 2019 Oct 21;58(20):13696-13708. doi: 10.1021/acs.inorgchem.9b01280. Epub 2019 Jul 31. Inorg Chem. 2019. PMID: 31364355 Free PMC article. Review.
-
Metal ion-dependent DNAzymes and their applications as biosensors.Met Ions Life Sci. 2012;10:217-48. doi: 10.1007/978-94-007-2172-2_8. Met Ions Life Sci. 2012. PMID: 22210341 Review.
-
DNAzyme sensors for detection of metal ions in the environment and imaging them in living cells.Curr Opin Biotechnol. 2017 Jun;45:191-201. doi: 10.1016/j.copbio.2017.03.002. Epub 2017 Apr 28. Curr Opin Biotechnol. 2017. PMID: 28458112 Free PMC article. Review.
Cited by
-
Intelligent demethylase-driven DNAzyme sensor for highly reliable metal-ion imaging in living cells.Chem Sci. 2021 Oct 29;12(46):15339-15346. doi: 10.1039/d1sc05370a. eCollection 2021 Dec 1. Chem Sci. 2021. PMID: 34976354 Free PMC article.
-
Imagining the future of optical microscopy: everything, everywhere, all at once.Commun Biol. 2023 Oct 28;6(1):1096. doi: 10.1038/s42003-023-05468-9. Commun Biol. 2023. PMID: 37898673 Free PMC article. Review.
-
Genetically encoded fluorescent sensors for metals in biology.Curr Opin Chem Biol. 2023 Jun;74:102284. doi: 10.1016/j.cbpa.2023.102284. Epub 2023 Mar 12. Curr Opin Chem Biol. 2023. PMID: 36917910 Free PMC article. Review.
-
On-Site Portable Lithium Detection in Mining and Recycling Industries Based on a DNAzyme Fluorescent Sensor.Angew Chem Int Ed Engl. 2025 Jan 10;64(2):e202413118. doi: 10.1002/anie.202413118. Epub 2024 Nov 29. Angew Chem Int Ed Engl. 2025. PMID: 39581875
-
Advancements and Prospects in DNA-Based Bioanalytical Technology for Environmental Toxicant Detection.JACS Au. 2025 Jun 2;5(6):2443-2462. doi: 10.1021/jacsau.5c00398. eCollection 2025 Jun 23. JACS Au. 2025. PMID: 40575295 Free PMC article. Review.
References
-
- Jiang P, Guo Z, Coord. Chem. Rev 2004, 248, 205–229;
- Kikuchi K, Komatsu K, Nagano T, Curr. Opin. Chem. Biol 2004, 8, 182–191; - PubMed
- Taki M, Wolford JL, O’Halloran TV, J. Am. Chem. Soc 2004, 126, 712–713; - PubMed
- Komatsu K, Urano Y, Kojima H, Nagano T, J. Am. Chem. Soc 2007, 129, 13447–13454; - PubMed
- Kennedy DP, Kormos CM, Burdette SC, J. Am. Chem. Soc 2009, 131, 8578–8586; - PubMed
- McRae R, Bagchi P, Sumalekshmy S, Fahrni CJ, Chem. Rev 2009, 109, 4780–4827; - PMC - PubMed
- Nolan EM, Lippard SJ, Acc. Chem. Res 2009, 42, 193–203; - PMC - PubMed
- Mikata Y, Kawata K, Takeuchi S, Nakanishi K, Konno H, Itami S, Yasuda K, Tamotsu S, Burdette SC, Dalton Trans. 2014, 43, 10751–10759; - PubMed
- Qiu L, Zhu C, Chen H, Hu M, He W, Guo Z, Chem. Commun 2014, 50, 4631–4634; - PubMed
- Sareen D, Kaur P, Singh K, Coord. Chem. Rev 2014, 265, 125–154;
- Cotruvo JA Jr., Aron AT, Ramos-Torres KM, Chang CJ, Chem. Soc. Rev 2015, 44, 4400–4414; - PMC - PubMed
- Aron AT, Loehr MO, Bogena J, Chang CJ, J. Am. Chem. Soc 2016, 138, 14338–14346; - PMC - PubMed
- Kaur N, Kaur P, Singh K, Sensor Actuat. B-Chem 2016, 229, 499–505;
- Hirata T, Terai T, Yamamura H, Shirnonishi M, Komatsu T, Hanaoka K, Ueno T, Imaizumi Y, Nagano T, Urano Y, Anal. Chem 2016, 88, 2693–2700; - PubMed
- Jia S, Ramos-Torres KM, Kolemen S, Ackerman CM, Chang CJ, ACS Chem. Biol 2018, 13, 1844–1852; - PMC - PubMed
- Bourassa D, Elitt CM, McCallum AM, Sumalekshmy S, McRae RL, Morgan MT, Siegel N, Perry JW, Rosenberg PA, Fahrni CJ, ACS Sensors 2018, 3, 458–467; - PMC - PubMed
- Goldberg JM, Wang F, Sessler CD, Vogler NW, Zhang DY, Loucks WH, Tzounopoulos T, Lippard SJ, J. Am. Chem. Soc 2018, 140, 2020–2023; - PMC - PubMed
- Matsui Y, Mizukami S, Kikuchi K, Chem. Lett 2018, 47, 23–26.
-
- Huang X, Meng J, Dong Y, Cheng Y, Zhu C, Polymer 2010, 51, 3064–3067.
-
- Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikurak M, Tsien RY, Nature 1997, 388, 882–887; - PubMed
- Outten CE, O’Halloran TV, Science 2001, 292, 2488–2492; - PubMed
- Chen P, He C, J. Am. Chem. Soc 2004, 126, 728–729; - PubMed
- Palmer AE, Jin C, Reed JC, Tsien RY, Proc. Natl. Acad. Sci. USA 2004, 101, 17404–17409; - PMC - PubMed
- Van Dongen EMWM, Dekkers LM, Spijker K, Meijer EW, Klomp LWJ, Merkx M, J. Am. Chem. Soc 2006, 128, 10754–10762; - PubMed
- Wegner SV, Okesli A, Chen P, He C, J. Am. Chem. Soc 2007, 129, 3474–3475; - PubMed
- Wallace DJ, Borgloh S, Astori S, Yang Y, Bausen M, Kugler S, Palmer AE, Tsien RY, Sprengel R, Kerr JND, Denk W, Hasan MT, Nat. Methods 2008, 5, 797–804; - PubMed
- Qin Y, Dittmer PJ, Park JG, Jansen KB, Palmer AE, Proc. Natl. Acad. Sci. USA 2011, 108, 7351–7356; - PMC - PubMed
- Lindenburg LH, Vinkenborg JL, Oortwijn J, Aper SJA, Merkx M, Plos One 2013, 8, e82009; - PMC - PubMed
- Carter KP, Young AM, Palmer AE, Chem. Rev 2014, 114, 4564–4601; - PMC - PubMed
- Hessels AM, Merkx M, Metallomics 2015, 7, 258–266; - PubMed
- Shen Y, Wu SY, Rancic V, Aggarwal A, Qian Y, Miyashita SI, Ballanyi K, Campbell RE, Dong M, Commun. Biol 2019, 2, 18. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

