Gas-Phase Sequencing of Cyclotides: Introduction of Selective Ring Opening at Dehydroalanine via Ion/Ion Reaction
- PMID: 31746593
- PMCID: PMC6917824
- DOI: 10.1021/acs.analchem.9b03671
Gas-Phase Sequencing of Cyclotides: Introduction of Selective Ring Opening at Dehydroalanine via Ion/Ion Reaction
Abstract
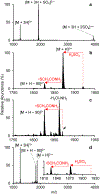
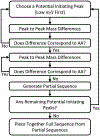
The gas-phase linearization of cyclotides via site-selective ring opening at dehydroalanine residues and its application to cyclotide sequencing is presented. This strategy relies on the ability to incorporate dehydroalanine into macrocyclic peptide ions, which is easily accomplished through an ion/ion reaction. Triply protonated cyclotide cations are transformed into radical cations via ion/ion reaction with the sulfate radical anion. Subsequent activation of the cyclotide radical cation generates dehydroalanine at a single cysteine residue, which is easily identified by the odd-electron loss of ·SCH2CONH2. The presence of dehydroalanine in cyclotides provides a site-selective ring-opening pathway that, in turn, generates linear cyclotide analogues in the gas phase. Unlike cyclic variants, product ions derived from the linear peptides provide rich sequence information. The sequencing capability of this strategy is demonstrated with four known cyclotides found in Viola inconspicua, where, in each case, greater than 93% sequence coverage was observed. Furthermore, the utility of this method is highlighted by the partial de novo sequencing of an unknown cyclotide with much greater sequence coverage than that obtained with a conventional Glu-C digestion approach. This method is particularly well-suited for cyclotide species that are not abundant enough to characterize with traditional methods.
Figures



Similar articles
-
Comprehensive Mapping of Cyclotides from Viola philippica by Using Mass Spectrometry-Based Strategy.Molecules. 2024 Sep 13;29(18):4344. doi: 10.3390/molecules29184344. Molecules. 2024. PMID: 39339338 Free PMC article.
-
Analysis of cyclotides in Viola ignobilis by Nano liquid chromatography fourier transform mass spectrometry.Protein Pept Lett. 2011 Jul;18(7):747-52. doi: 10.2174/092986611795446030. Protein Pept Lett. 2011. PMID: 21413917
-
Reporting a Transcript from Iranian Viola Tricolor, Which May Encode a Novel Cyclotide-Like Precursor: Molecular and in silico Studies.Comput Biol Chem. 2020 Feb;84:107168. doi: 10.1016/j.compbiolchem.2019.107168. Epub 2019 Nov 21. Comput Biol Chem. 2020. PMID: 31791808
-
Novel strategies for isolation and characterization of cyclotides: the discovery of bioactive macrocyclic plant polypeptides in the Violaceae.Curr Protein Pept Sci. 2004 Oct;5(5):317-29. doi: 10.2174/1389203043379495. Curr Protein Pept Sci. 2004. PMID: 15544528 Review.
-
Cyclotides: Disulfide-rich peptide toxins in plants.Toxicon. 2019 Oct 25;172:33-44. doi: 10.1016/j.toxicon.2019.10.244. Epub 2019 Nov 1. Toxicon. 2019. PMID: 31682883 Review.
Cited by
-
2-Pyridine Carboxaldehyde for Semi-Automated Soft Spot Identification in Cyclic Peptides.Int J Mol Sci. 2022 Apr 12;23(8):4269. doi: 10.3390/ijms23084269. Int J Mol Sci. 2022. PMID: 35457087 Free PMC article.
-
Leveraging orthogonal mass spectrometry based strategies for comprehensive sequencing and characterization of ribosomal antimicrobial peptide natural products.Nat Prod Rep. 2021 Mar 1;38(3):489-509. doi: 10.1039/d0np00046a. Epub 2020 Sep 15. Nat Prod Rep. 2021. PMID: 32929442 Free PMC article. Review.
-
Application of chloroplast genome in the identification of Traditional Chinese Medicine Viola philippica.BMC Genomics. 2022 Jul 27;23(1):540. doi: 10.1186/s12864-022-08727-x. BMC Genomics. 2022. PMID: 35896957 Free PMC article.
References
-
- Craik DJ, Daly NL, Bond T, Waine C: Plant cyclotides: A unique family of cyclic and knotted proteins that defines the cyclic cystine knot structural motif. J. Mol. Biol 294, 1327–1336 (1999) - PubMed
-
- Colgrave ML, Craik DJ: Thermal, Chemical, and Enzymatic Stability of the Cyclotide Kalata B1: The Importance of the Cyclic Cystine Knot. Biochemistry. 43, 5965–5975 (2004) - PubMed
-
- Henriques ST, Craik DJ: Cyclotides as templates in drug design. Drug Discov. Today 15, 57–64 (2010) - PubMed
-
- Poth AG, Chan LY, Craik DJ: Cyclotides as grafting frameworks for protein engineering and drug design applications. J. Pept. Sci 100, 480–491 (2013) - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

