Design, Synthesis, and Study of Lactam and Ring-Expanded Analogues of Teixobactin
- PMID: 31746604
- PMCID: PMC7814697
- DOI: 10.1021/acs.joc.9b02631
Design, Synthesis, and Study of Lactam and Ring-Expanded Analogues of Teixobactin
Abstract
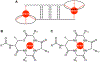
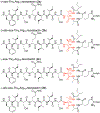
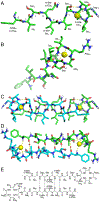
This paper describes the chemical synthesis, X-ray crystallographic structure, and antibiotic activity assay of lactam analogues of teixobactin and explores ring-expanded analogues of teixobactin with β3-homo amino acids. Lactam analogues of teixobactin containing all four stereoisomers of aza-threonine at position 8 were synthesized on a solid support from commercially available stereoisomeric threonine derivatives. The threonine stereoisomers are converted to the diastereomeric aza-threonines by mesylation, azide displacement, and reduction during the synthesis. d-Aza-Thr8,Arg10-teixobactin exhibits 2-8-fold greater antibiotic activity than the corresponding macrolactone Arg10-teixobactin. Azateixobactin analogues containing other stereoisomers of aza-threonine are inactive. A dramatic 16-128-fold increase in the activity of teixobactin and teixobactin analogues is observed with the inclusion of 0.002% of the mild detergent polysorbate 80 in the MIC assay. The X-ray crystallographic structure of N-Me-d-Gln4,d-aza-Thr8,Arg10-teixobactin reveals an amphipathic hydrogen-bonded antiparallel β-sheet dimer that binds chloride anions. In the binding site, the macrolactam amide NH groups of residues 8, 10, and 11, as well as the extra amide NH group of the lactam ring, hydrogen bond to the chloride anion. The teixobactin pharmacophore tolerates ring expansion of the 13-membered ring to 14-,15-, and 16-membered rings containing β3-homo amino acids with retention of partial or full antibiotic activity.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Ling LL; Schneider T; Peoples AJ; Spoering AL; Engels I; Conlon BP; Mueller A; Schäberle TF; Hughes DE; Epstein S; Jones M; Lazarides L; Steadman VA; Cohen DR; Felix CR; Fetterman KA; Millett WP; Nitti AG; Zullo AM; Chen C; Lewis K A New Antibiotic Kills Pathogens Without Detectable Resistance. Nature 2015, 517, 455–459. - PMC - PubMed
-
- Breukink E; de Kruijff B Lipid II as a Target for Antibiotics. Nat. Rev. Drug Discov 2006, 5, 321–323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

