Particulate matter and episodic memory decline mediated by early neuroanatomic biomarkers of Alzheimer's disease
- PMID: 31746986
- PMCID: PMC6938036
- DOI: 10.1093/brain/awz348
Particulate matter and episodic memory decline mediated by early neuroanatomic biomarkers of Alzheimer's disease
Erratum in
-
Erratum.Brain. 2020 Mar 1;143(3):e24. doi: 10.1093/brain/awaa007. Brain. 2020. PMID: 32333675 Free PMC article. No abstract available.
Abstract
Evidence suggests exposure to particulate matter with aerodynamic diameter <2.5 μm (PM2.5) may increase the risk for Alzheimer's disease and related dementias. Whether PM2.5 alters brain structure and accelerates the preclinical neuropsychological processes remains unknown. Early decline of episodic memory is detectable in preclinical Alzheimer's disease. Therefore, we conducted a longitudinal study to examine whether PM2.5 affects the episodic memory decline, and also explored the potential mediating role of increased neuroanatomic risk of Alzheimer's disease associated with exposure. Participants included older females (n = 998; aged 73-87) enrolled in both the Women's Health Initiative Study of Cognitive Aging and the Women's Health Initiative Memory Study of Magnetic Resonance Imaging, with annual (1999-2010) episodic memory assessment by the California Verbal Learning Test, including measures of immediate free recall/new learning (List A Trials 1-3; List B) and delayed free recall (short- and long-delay), and up to two brain scans (MRI-1: 2005-06; MRI-2: 2009-10). Subjects were assigned Alzheimer's disease pattern similarity scores (a brain-MRI measured neuroanatomical risk for Alzheimer's disease), developed by supervised machine learning and validated with data from the Alzheimer's Disease Neuroimaging Initiative. Based on residential histories and environmental data on air monitoring and simulated atmospheric chemistry, we used a spatiotemporal model to estimate 3-year average PM2.5 exposure preceding MRI-1. In multilevel structural equation models, PM2.5 was associated with greater declines in immediate recall and new learning, but no association was found with decline in delayed-recall or composite scores. For each interquartile increment (2.81 μg/m3) of PM2.5, the annual decline rate was significantly accelerated by 19.3% [95% confidence interval (CI) = 1.9% to 36.2%] for Trials 1-3 and 14.8% (4.4% to 24.9%) for List B performance, adjusting for multiple potential confounders. Long-term PM2.5 exposure was associated with increased Alzheimer's disease pattern similarity scores, which accounted for 22.6% (95% CI: 1% to 68.9%) and 10.7% (95% CI: 1.0% to 30.3%) of the total adverse PM2.5 effects on Trials 1-3 and List B, respectively. The observed associations remained after excluding incident cases of dementia and stroke during the follow-up, or further adjusting for small-vessel ischaemic disease volumes. Our findings illustrate the continuum of PM2.5 neurotoxicity that contributes to early decline of immediate free recall/new learning at the preclinical stage, which is mediated by progressive atrophy of grey matter indicative of increased Alzheimer's disease risk, independent of cerebrovascular damage.
Keywords: Alzheimer’s disease; air pollution; episodic memory; fine particulate matter; neuroimaging.
© The Author(s) (2019). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

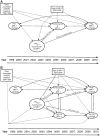
Comment in
-
Growing evidence links air pollution exposure to risk of Alzheimer's disease and related dementia.Brain. 2020 Jan 1;143(1):8-10. doi: 10.1093/brain/awz396. Brain. 2020. PMID: 31886492 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- HHSN268201100001I/HL/NHLBI NIH HHS/United States
- P50 AG005142/AG/NIA NIH HHS/United States
- R21 AG051113/AG/NIA NIH HHS/United States
- HHSN268201100004I/HL/NHLBI NIH HHS/United States
- HHSN268201100046C/HL/NHLBI NIH HHS/United States
- K23 AG057760/AG/NIA NIH HHS/United States
- HHSN271201100004C/AG/NIA NIH HHS/United States
- HHSN268201100002C/WH/WHI NIH HHS/United States
- P50 AG047366/AG/NIA NIH HHS/United States
- HHSN268201100002I/HL/NHLBI NIH HHS/United States
- HHSN268201100001C/WH/WHI NIH HHS/United States
- HHSN268201100004C/WH/WHI NIH HHS/United States
- N01 WH044221/WH/WHI NIH HHS/United States
- P30 ES010126/ES/NIEHS NIH HHS/United States
- P30 AG066507/AG/NIA NIH HHS/United States
- P30 ES007048/ES/NIEHS NIH HHS/United States
- HHSN268201100003C/WH/WHI NIH HHS/United States
- R01 ES025888/ES/NIEHS NIH HHS/United States
- P01 AG055367/AG/NIA NIH HHS/United States
- RF1 AG054068/AG/NIA NIH HHS/United States
- P30 AG049638/AG/NIA NIH HHS/United States
- P30 ES007033/ES/NIEHS NIH HHS/United States
- P30 AG066530/AG/NIA NIH HHS/United States
- R01 AG033078/AG/NIA NIH HHS/United States
- HHSN268201100003I/HL/NHLBI NIH HHS/United States

