An Evaluation of US Food and Drug Administration's Program to Register HIV Drugs for Use in Resource-Constrained Settings
- PMID: 31747034
- PMCID: PMC6902815
- DOI: 10.1001/jamanetworkopen.2019.15787
An Evaluation of US Food and Drug Administration's Program to Register HIV Drugs for Use in Resource-Constrained Settings
Abstract
Importance: The US Food and Drug Administration (FDA) program to review antiretroviral drugs for use in low-resource settings via the US President's Emergency Plan for AIDS Relief (PEPFAR) now supports treatment of more than 14 million patients with HIV. However, an in-depth evaluation of the program has not been undertaken.
Objective: To conduct a quantitative analysis of the FDA-reviewed antiretroviral drug applications in order to assess the contributions of PEPFAR and to identify areas for improvement.
Design, setting, and participants: A cross-sectional study was conducted of all PEPFAR applications submitted to the FDA from December 1, 2004, to May 31, 2018. The analyses were conducted between October 2018 and February 2019.
Main outcomes and measures: Numbers and types of applications reviewed, how long it took for applications to obtain approval or tentative approval (time to registration), how often the FDA issued a complete response letter (CRL) identifying deficiencies precluding application approval or tentative approval and their reasons, and the association between CRLs and time to registration.
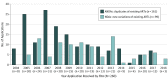
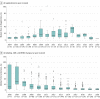
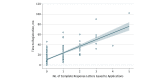
Results: Overall, 260 PEPFAR applications for 327 antiretroviral therapies were reviewed by FDA, of which 216 applications (83%) for 272 drugs were authorized for use. Of the 216 authorized applications, 184 applications for 231 drugs remain in active status and, thus, are available for use. Twenty-six percent (56 of 216) of the applications were for pediatric-specific formulations or strengths; the remainder were for adults. For all 216 applications, the median (interquartile range) time to registration was 10.0 (7.0-17.5) months. Thirty-seven percent (95 of 260) of the applications received 1 or more CRLs, resulting in a total of 172 CRLs; most applications received 1 CRL, whereas some were issued up to 6 CRLs. Among all CRLs, 264 deficiency reasons were identified; the most common deficiencies were associated with manufacturing processes (155 [44%]), followed by product labeling (62 [23%]), and failing facility inspections (54 [20%]). Complete response letters were associated with an increased time to registration. Applications without CRLs had a median (interquartile range) time to registration of 9.0 (5.5-12.0) months, whereas those with at least 1 CRL took a median (interquartile range) of 22.0 (14.0-38.0) months (P < .001).
Conclusions and relevance: The FDA's PEPFAR program has made many antiretroviral drugs available for global use. However, FDA and the pharmaceutical companies could take steps to improve the quality of applications submitted to prevent avoidable deficiencies in manufacturing processes and labeling. Further efforts to develop better, easier to use pediatric-specific therapies are needed.
Conflict of interest statement
Figures
References
-
- Joint United Nations Programme on HIV/AIDS (UNAIDS) Global HIV & AIDS statistics—2019 fact sheet. https://www.unaids.org/en/resources/fact-sheet. Accessed September 23, 2019.
-
- Institute of Medicine Design Considerations for Evaluating the Impact of PEPFAR: Workshop Summary (2008). Washington, DC: National Academies Press; 2008. - PubMed
-
- US Department of State PEPFAR: the United States President’s Emergency Plan for AIDS Relief. https://www.state.gov/pepfar/. Published 2018. Accessed November 3, 2018.
-
- US Food and Drug Administration Fixed Dose Combinations, Co-Packaged Drug Products, and Single-Entity Versions of Previously Approved Antiretrovirals for the Treatment of HIV. Rockville, MD: US Department of Health and Human Services; 2006.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

