The Multifaceted Inhibitory Effects of an Alkylquinolone on the Diatom Phaeodactylum tricornutum
- PMID: 31747114
- PMCID: PMC7217009
- DOI: 10.1002/cbic.201900612
The Multifaceted Inhibitory Effects of an Alkylquinolone on the Diatom Phaeodactylum tricornutum
Abstract
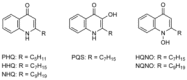
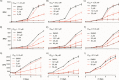
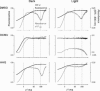
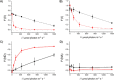
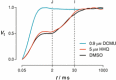
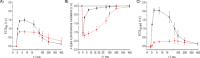
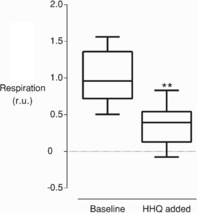
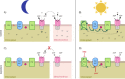
The mechanisms underlying interactions between diatoms and bacteria are crucial to understand diatom behaviour and proliferation, and can result in far-reaching ecological consequences. Recently, 2-alkyl-4-quinolones have been isolated from marine bacteria, both of which (the bacterium and isolated chemical) inhibited growth of microalgae, suggesting these compounds could mediate diatom-bacteria interactions. The effects of several quinolones on three diatom species have been investigated. The growth of all three was inhibited, with half-maximal inhibitory concentrations reaching the sub-micromolar range. By using multiple techniques, dual inhibition mechanisms were uncovered for 2-heptyl-4-quinolone (HHQ) in Phaeodactylum tricornutum. Firstly, photosynthetic electron transport was obstructed, primarily through inhibition of the cytochrome b6 f complex. Secondly, respiration was inhibited, leading to repression of ATP supply to plastids from mitochondria through organelle energy coupling. These data clearly show how HHQ could modulate diatom proliferation in marine environments.
Keywords: cytochromes; diatom-bacteria interactions; photosynthesis; quinolones; reaction mechanisms.
© 2019 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

