Structural Identity of Galactooligosaccharide Molecules Selectively Utilized by Single Cultures of Probiotic Bacterial Strains
- PMID: 31747272
- PMCID: PMC6923793
- DOI: 10.1021/acs.jafc.9b05968
Structural Identity of Galactooligosaccharide Molecules Selectively Utilized by Single Cultures of Probiotic Bacterial Strains
Abstract
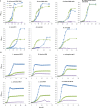
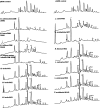
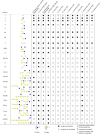
Various β-galactosidase enzymes catalyze the trans-glycosylation reaction with lactose. The resulting galactooligosaccharide (GOS) mixtures are widely used in infant nutrition to stimulate growth of beneficial gut bacteria. GOS consists mainly of compounds with a degree of polymerization (DP) varying from 2-8 and with diverse glycosidic linkages. In recent years, we have elucidated in detail the composition of several commercial GOS mixtures in terms of DP and the structural identity of the individual compounds. In this work, 13 (single) probiotic strains of gut bacteria, belonging to 11 different species, were grown to stationary phase with a Vivinal GOS-derived sample purified to remove lactose and monosaccharides (pGOS). Growth among the probiotic strains varied strongly between 30 and 100% of OD600nm relative to positive controls with glucose. By identifying the components of the pGOS mixture that remain after growth, we showed that strains varied in their consumption of specific GOS compounds. All strains commonly used most of the GOS DP2 pool. Lactobacillus salivarius W57 also utilized the DP3 branched compound β-d-Galp-(1 → 4)-[β-d-Galp-(1 → 2)]-d-Glc. Bifidobacterial strains tended to use GOS with higher DP and branching than lactobacilli; Bifidobacterium breve DSM 20091, Lactobacillus acidophilus W37, and Bifidobacterium infantis DSM 20088 were exceptional in using 38, 36, and 35 compounds, respectively, out of the 40 different structures identified in pGOS. We correlated these bacterial GOS consumption profiles with their genomic information and were able to relate metabolic activity with the presence of genome-encoded transporters and carbohydrate-active enzymes. These detailed insights may support the design of synbiotic combinations pairing probiotic bacterial strains with GOS compounds that specifically stimulate their growth. Such synbiotic combinations may be of interest in food/feed and/or pharmacy/medicine applications.
Keywords: bifidobacteria; catabolic pathways; galactooligosaccharides; glycosidic linkages; lactic acid bacteria; synbiotics.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Krogsgaard L. R.; Andersen L. O.; Johannesen T. B.; Engsbro A. L.; Stensvold C. R.; Nielsen H. V.; Bytzer P. Characteristics of the bacterial microbiome in association with common intestinal parasites in irritable bowel syndrome. Clin. Transl. Gastroenterol. 2018, 9, e16110.1038/s41424-018-0027-2. - DOI - PMC - PubMed
-
- Kochhar S.; Martin F.. Metabonomics and Gut Microbiota in Nutrition and Disease; Springer: London, 2015.
-
- Gibson G. R.; Hutkins R.; Sanders M. E.; Prescott S. L.; Reimer R. A.; Salminen S. J.; Scott K.; Stanton C.; Swanson K. S.; Cani P. D.; Verbeke K.; Reid G. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. 10.1038/nrgastro.2017.75. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

