Binocular 3D otolith-ocular reflexes: responses of normal chinchillas to tilt and translation
- PMID: 31747360
- PMCID: PMC6985861
- DOI: 10.1152/jn.00882.2018
Binocular 3D otolith-ocular reflexes: responses of normal chinchillas to tilt and translation
Abstract
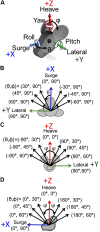
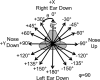
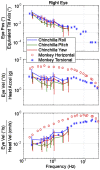
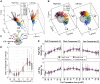
Head rotation, translation, and tilt with respect to a gravitational field elicit reflexive eye movements that partially stabilize images of Earth-fixed objects on the retinas of humans and other vertebrates. Compared with the angular vestibulo-ocular reflex, responses to translation and tilt, collectively called the otolith-ocular reflex (OOR), are less completely characterized, typically smaller, generally disconjugate (different for the 2 eyes) and more complicated in their relationship to the natural stimuli that elicit them. We measured binocular 3-dimensional OOR responses of 6 alert normal chinchillas in darkness during whole body tilts around 16 Earth-horizontal axes and translations along 21 axes in horizontal, coronal, and sagittal planes. Ocular countertilt responses to 40-s whole body tilts about Earth-horizontal axes grew linearly with head tilt amplitude, but responses were disconjugate, with each eye's response greatest for whole body tilts about axes near the other eye's resting line of sight. OOR response magnitude during 1-Hz sinusoidal whole body translations along Earth-horizontal axes also grew with stimulus amplitude. Translational OOR responses were similarly disconjugate, with each eye's response greatest for whole body translations along its resting line of sight. Responses to Earth-horizontal translation were similar to those that would be expected for tilts that would cause a similar peak deviation of the gravitoinertial acceleration (GIA) vector with respect to the head, consistent with the "perceived tilt" model of the OOR. However, that model poorly fit responses to translations along non-Earth-horizontal axes and was insufficient to explain why responses are larger for the eye toward which the GIA vector deviates.NEW & NOTEWORTHY As the first in a pair of papers on Binocular 3D Otolith-Ocular Reflexes, this paper characterizes binocular 3D eye movements in normal chinchillas during tilts and translations. The eye movement responses were used to create a data set to fully define the normal otolith-ocular reflexes in chinchillas. This data set provides the foundation to use otolith-ocular reflexes to back-project direction and magnitude of eye movement to predict tilt axis as discussed in the companion paper.
Keywords: otolith-ocular reflex; saccule; utricle; vestibular; vestibulo-ocular reflex.
Conflict of interest statement
C. C. Della Santina holds an equity interest in Labyrinth Devices, LLC, which is working to develop a vestibular implant for clinical use through a collaboration with Johns Hopkins School of Medicine and Med-El, GmbH. The terms of that arrangement are managed in accordance with John Hopkins University policies on conflicts of interest and interaction with industry.
Figures






Similar articles
-
Binocular 3D otolith-ocular reflexes: responses of chinchillas to prosthetic electrical stimulation targeting the utricle and saccule.J Neurophysiol. 2020 Jan 1;123(1):259-276. doi: 10.1152/jn.00883.2018. Epub 2019 Nov 20. J Neurophysiol. 2020. PMID: 31747349 Free PMC article.
-
Binocular 3-D otolith-ocular reflexes: responses of chinchillas to natural and prosthetic stimulation after ototoxic injury and vestibular implantation.J Neurophysiol. 2023 May 1;129(5):1157-1176. doi: 10.1152/jn.00445.2022. Epub 2023 Apr 5. J Neurophysiol. 2023. PMID: 37018758 Free PMC article.
-
Three-dimensional organization of otolith-ocular reflexes in rhesus monkeys. I. Linear acceleration responses during off-vertical axis rotation.J Neurophysiol. 1996 Jun;75(6):2405-24. doi: 10.1152/jn.1996.75.6.2405. J Neurophysiol. 1996. PMID: 8793753
-
New understanding on the contribution of the central otolithic system to eye movement and skew deviation.Eye (Lond). 2015 Feb;29(2):153-6. doi: 10.1038/eye.2014.243. Epub 2014 Oct 17. Eye (Lond). 2015. PMID: 25323854 Free PMC article. Review.
-
Adaptive plasticity in the otolith-ocular reflex.Auris Nasus Larynx. 2003 Feb;30 Suppl:S3-6. doi: 10.1016/s0385-8146(02)00117-7. Auris Nasus Larynx. 2003. PMID: 12543152 Review.
Cited by
-
Binocular 3D otolith-ocular reflexes: responses of chinchillas to prosthetic electrical stimulation targeting the utricle and saccule.J Neurophysiol. 2020 Jan 1;123(1):259-276. doi: 10.1152/jn.00883.2018. Epub 2019 Nov 20. J Neurophysiol. 2020. PMID: 31747349 Free PMC article.
-
Binocular 3-D otolith-ocular reflexes: responses of chinchillas to natural and prosthetic stimulation after ototoxic injury and vestibular implantation.J Neurophysiol. 2023 May 1;129(5):1157-1176. doi: 10.1152/jn.00445.2022. Epub 2023 Apr 5. J Neurophysiol. 2023. PMID: 37018758 Free PMC article.
-
Clinical Research on Positron Emission Tomography Imaging of the Neuro-Stimulation System in Patients with Cochleo-Vestibular Implants: Is There a Response Beyond the Peripheral Organ?J Clin Med. 2025 Feb 21;14(5):1445. doi: 10.3390/jcm14051445. J Clin Med. 2025. PMID: 40094915 Free PMC article.
-
An Implanted Vestibular Prosthesis Improves Spatial Orientation in Animals with Severe Vestibular Damage.J Neurosci. 2021 Apr 28;41(17):3879-3888. doi: 10.1523/JNEUROSCI.2204-20.2021. Epub 2021 Mar 17. J Neurosci. 2021. PMID: 33731447 Free PMC article.
References
-
- Boutros PJ, Schoo DP, Rahman M, Valentin NS, Chow MR, Ayiotis AI, Morris BJ, Hofner A, Rascon AM, Marx A, Deas R, Fridman GY, Davidovics NS, Ward BK, Treviño C, Bowditch SP, Roberts DC, Lane KE, Gimmon Y, Schubert MC, Carey JP, Jaeger A, Della Santina CC. Continuous vestibular implant stimulation partially restores eye-stabilizing reflexes. JCI Insight 4: 128397, 2019. doi:10.1172/jci.insight.128397. - DOI - PMC - PubMed
-
- Carey JP, Della Santina C. Principles of applied vestibular physiology. In: Cummings Otolaryngology: Head & Neck Surgery. Philadelphia, PA: Mosby, 2005, p. 3115–3159.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

