Definitive radiochemotherapy in esophageal cancer - a single institution experience
- PMID: 31747382
- PMCID: PMC6884939
- DOI: 10.2478/raon-2019-0054
Definitive radiochemotherapy in esophageal cancer - a single institution experience
Abstract
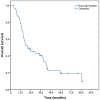
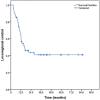
Background Definitive radiochemotherapy is the preferred treatment option in patients with the cancer of the cervical esophagus and a viable treatment option in patients with the cancer of lower two thirds of the esophagus, who decline proposed surgical treatment. The purpose of the study was to evaluate the treatment results with definitive radiochemotherapy of patients with esophageal cancer, treated in a single institution in the period from 2010 to 2017. Patients and methods All available medical data for 55 patients with esophageal cancer, who were treated with definitive radiochemotherapy with curative intent, were analyzed retrospectively. Patients were irradiated to a total dose to the tumor of 70 Gy (2 Gy per fraction) in upper third (cervical) tumors or to the mean total dose of 57.6 Gy (1.8 Gy per fraction) in middle third (intrathoracic) tumors. All but one patient received concomitant chemotherapy, with the majority of them (41 patients; 74.5%) receiving concomitant chemotherapy with 5-fluorouracil in continuous 96 hours infusion and cisplatin. The main endpoints of the study were overall survival (OS; death of any cause), locoregional control (LRC; local and/or regional disease recurrence) and disease-free survival (DFS; recurrence of any kind and/or new primary malignoma). Univariate analysis testing the impact of different parameters on survivals and analysis of treatment related side effects were performed as well. Results The mean age of patients was 62 years (SD 9 years; range: 29-80 years). Majority of them had squamous cell cancer (53 patients; 96.4%) in the stage T3 or T4 (47 patients; 85.5%) and/or N+ disease (35 patients; 63.6%). Median follow-up time for the whole group of patients was 16.8 months (range: 0.3-81.8 months). At the time of analysis 14 (25.5%) patients were still alive. Rates for OS, LRC and DFS at two and five years were as follows: 47% and 19.4%; 43.7% and 41%; 32.1% and 11.5%, respectively. Conclusions The study results of treatment with definitive radiochemotherapy in patients with esophageal cancer are similar to the results of other studies. Majority of patients ended the treatment according to the protocol, which at least in part can be attributed to the adequate and well organized supportive treatment in our institution.
Keywords: definitive radiochemotherapy; esophageal cancer; loco-regional control; survival.
Figures
References
-
- NCCN clinical practice guidelines in oncology (NCCN Guidelines®) 2019. https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf Esophageal and esophagogastric junction cancers, version 1. [cited 2019 Jan 15]. Available at. - PubMed
-
- Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA, Al-Sarraf M. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623–7. doi: 10.1001/jama.281.17.1623. et al. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical