ASL Metabolically Regulates Tyrosine Hydroxylase in the Nucleus Locus Coeruleus
- PMID: 31747589
- PMCID: PMC6902269
- DOI: 10.1016/j.celrep.2019.10.043
ASL Metabolically Regulates Tyrosine Hydroxylase in the Nucleus Locus Coeruleus
Abstract
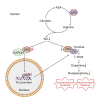
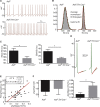
Patients with germline mutations in the urea-cycle enzyme argininosuccinate lyase (ASL) are at risk for developing neurobehavioral and cognitive deficits. We find that ASL is prominently expressed in the nucleus locus coeruleus (LC), the central source of norepinephrine. Using natural history data, we show that individuals with ASL deficiency are at risk for developing attention deficits. By generating LC-ASL-conditional knockout (cKO) mice, we further demonstrate altered response to stressful stimuli with increased seizure reactivity in LC-ASL-cKO mice. Depletion of ASL in LC neurons leads to reduced amount and activity of tyrosine hydroxylase (TH) and to decreased catecholamines synthesis, due to decreased nitric oxide (NO) signaling. NO donors normalize catecholamine levels in the LC, seizure sensitivity, and the stress response in LC-ASL-cKO mice. Our data emphasize ASL importance for the metabolic regulation of LC function with translational relevance for ASL deficiency (ASLD) patients as well as for LC-related pathologies.
Keywords: ASL; locus coeruleus; nitric oxide; stress response; tyrosine hydroxylase; urea cycle disorders.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Anderzhanova E.A., Bächli H., Buneeva O.A., Narkevich V.B., Medvedev A.E., Thoeringer C.K., Wotjak C.T., Kudrin V.S. Strain differences in profiles of dopaminergic neurotransmission in the prefrontal cortex of the BALB/C vs. C57Bl/6 mice: consequences of stress and afobazole. Eur. J. Pharmacol. 2013;708:95–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

