A new antibiotic selectively kills Gram-negative pathogens
- PMID: 31747680
- PMCID: PMC7188312
- DOI: 10.1038/s41586-019-1791-1
A new antibiotic selectively kills Gram-negative pathogens
Erratum in
-
Author Correction: A new antibiotic selectively kills Gram-negative pathogens.Nature. 2020 Apr;580(7802):E3. doi: 10.1038/s41586-020-2063-9. Nature. 2020. PMID: 32269338
Abstract
The current need for novel antibiotics is especially acute for drug-resistant Gram-negative pathogens1,2. These microorganisms have a highly restrictive permeability barrier, which limits the penetration of most compounds3,4. As a result, the last class of antibiotics that acted against Gram-negative bacteria was developed in the 1960s2. We reason that useful compounds can be found in bacteria that share similar requirements for antibiotics with humans, and focus on Photorhabdus symbionts of entomopathogenic nematode microbiomes. Here we report a new antibiotic that we name darobactin, which was obtained using a screen of Photorhabdus isolates. Darobactin is coded by a silent operon with little production under laboratory conditions, and is ribosomally synthesized. Darobactin has an unusual structure with two fused rings that form post-translationally. The compound is active against important Gram-negative pathogens both in vitro and in animal models of infection. Mutants that are resistant to darobactin map to BamA, an essential chaperone and translocator that folds outer membrane proteins. Our study suggests that bacterial symbionts of animals contain antibiotics that are particularly suitable for development into therapeutics.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





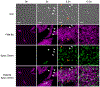
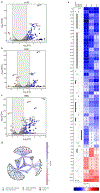


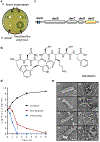



Comment in
-
New drugs for the antibacterial pipeline?Nat Rev Microbiol. 2020 Feb;18(2):61. doi: 10.1038/s41579-019-0310-6. Nat Rev Microbiol. 2020. PMID: 31797908 No abstract available.
-
New antibiotics target the outer membrane of bacteria.Nature. 2019 Dec;576(7787):389-390. doi: 10.1038/d41586-019-03730-x. Nature. 2019. PMID: 31844257 No abstract available.
-
Learning from worms to kill Gram-negative bacteria.Nat Rev Drug Discov. 2020 Jan;19(1):22. doi: 10.1038/d41573-019-00208-3. Nat Rev Drug Discov. 2020. PMID: 31907429 No abstract available.
References
-
- Payne DJ, Gwynn MN, Holmes DJ & Pompliano DL Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat Rev Drug Discov 6, 29–40 (2007) - PubMed
-
- Lewis K Platforms for antibiotic discovery. Nat Rev Drug Discov 12, 371–387 (2013) - PubMed
-
- Li XZ & Nikaido H Efflux-mediated drug resistance in bacteria. Drugs 64, 159–204 (2004) - PubMed
-
- Tacconelli E et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 18, 318–327 (2018) - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

