Mechanism of modulation of AMPA receptors by TARP-γ8
- PMID: 31748249
- PMCID: PMC7034100
- DOI: 10.1085/jgp.201912451
Mechanism of modulation of AMPA receptors by TARP-γ8
Abstract
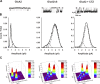
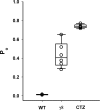
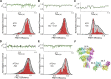
Fast excitatory synaptic transmission in the mammalian central nervous system is mediated by glutamate-activated α-amino-5-methyl-3-hydroxy-4-isoxazole propionate (AMPA) receptors. In neurons, AMPA receptors coassemble with transmembrane AMPA receptor regulatory proteins (TARPs). Assembly with TARP γ8 alters the biophysical properties of the receptor, producing resensitization currents in the continued presence of glutamate. Using single-channel recordings, we show that under resensitizing conditions, GluA2 AMPA receptors primarily transition to higher conductance levels, similar to activation of the receptors in the presence of cyclothiazide, which stabilizes the open state. To study the conformation associated with these states, we have used single-molecule FRET and show that this high-conductance state exhibits tighter coupling between subunits in the extracellular parts of the receptor. Furthermore, the dwell times for the transition from the tightly coupled state to the decoupled states correlate to longer open durations of the channels, thus correlating conformation and function at the single-molecule level.
© 2019 Carrillo et al.
Figures







Comment in
-
Resensitizing AMPA receptors.J Gen Physiol. 2020 Jan 6;152(1):e201912542. doi: 10.1085/jgp.201912542. J Gen Physiol. 2020. PMID: 31825464 Free PMC article.

