A proteolytic C-terminal fragment of Nogo-A (reticulon-4A) is released in exosomes and potently inhibits axon regeneration
- PMID: 31748413
- PMCID: PMC7039549
- DOI: 10.1074/jbc.RA119.009896
A proteolytic C-terminal fragment of Nogo-A (reticulon-4A) is released in exosomes and potently inhibits axon regeneration
Abstract
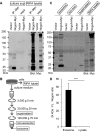
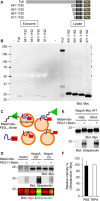
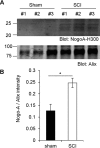
Glial signals are known to inhibit axonal regeneration and functional recovery after mammalian central nervous system trauma, including spinal cord injury. Such signals include membrane-associated proteins of the oligodendrocyte plasma membrane and astrocyte-derived, matrix-associated proteins. Here, using cell lines and primary cortical neuron cultures, recombinant protein expression, immunoprecipitation and immunoblot assays, transmission EM of exosomes, and axon regeneration assays, we explored the secretion and activity of the myelin-associated neurite outgrowth inhibitor Nogo-A and observed exosomal release of a 24-kDa C-terminal Nogo-A fragment from cultured cells. We found that the cleavage site in this 1192-amino-acid-long fragment is located between amino acids 961-971. We also detected a Nogo-66 receptor (NgR1)-interacting Nogo-66 domain on the exosome surface. Enzyme inhibitor treatment and siRNA knockdown revealed that β-secretase 1 (BACE1) is the protease responsible for Nogo-A cleavage. Functionally, exosomes with the Nogo-66 domain on their surface potently inhibited axonal regeneration of mechanically injured cerebral cortex neurons from mice. Production of this fragment was observed in the exosomal fraction from neuronal tissue lysates after spinal cord crush injury of mice. We also noted that, relative to the exosomal marker Alix, a Nogo-immunoreactive, 24-kDa protein is enriched in exosomes 2-fold after injury. We conclude that membrane-associated Nogo-A produced in oligodendrocytes is processed proteolytically by BACE1, is released via exosomes, and is a potent diffusible inhibitor of regenerative growth in NgR1-expressing axons.
Keywords: NgR1; Nogo; Nogo receptor; axon; exosome (vesicle); oligodendrocyte; regeneration; reticulon; spinal cord injury; β-secretase 1 (BACE1).
© 2020 Sekine et al.
Conflict of interest statement
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. S.M.S. is a cofounder of ReNetX Bio, which seeks to develop NgR1-based therapeutics
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

