Distinct CD1d docking strategies exhibited by diverse Type II NKT cell receptors
- PMID: 31748533
- PMCID: PMC6868179
- DOI: 10.1038/s41467-019-12941-9
Distinct CD1d docking strategies exhibited by diverse Type II NKT cell receptors
Abstract
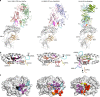
Type I and type II natural killer T (NKT) cells are restricted to the lipid antigen-presenting molecule CD1d. While we have an understanding of the antigen reactivity and function of type I NKT cells, our knowledge of type II NKT cells in health and disease remains unclear. Here we describe a population of type II NKT cells that recognise and respond to the microbial antigen, α-glucuronosyl-diacylglycerol (α-GlcADAG) presented by CD1d, but not the prototypical type I NKT cell agonist, α-galactosylceramide. Surprisingly, the crystal structure of a type II NKT TCR-CD1d-α-GlcADAG complex reveals a CD1d F'-pocket-docking mode that contrasts sharply with the previously determined A'-roof positioning of a sulfatide-reactive type II NKT TCR. Our data also suggest that diverse type II NKT TCRs directed against distinct microbial or mammalian lipid antigens adopt multiple recognition strategies on CD1d, thereby maximising the potential for type II NKT cells to detect different lipid antigens.
Conflict of interest statement
D.I.G. is a member of the scientific advisory board for Avalia Immunotherapies, a company that is developing NKT cell-based vaccines. The other authors declare no competing interests.
Figures



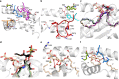

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

