A sodium-ion sulfide solid electrolyte with unprecedented conductivity at room temperature
- PMID: 31748566
- PMCID: PMC6868223
- DOI: 10.1038/s41467-019-13178-2
A sodium-ion sulfide solid electrolyte with unprecedented conductivity at room temperature
Abstract
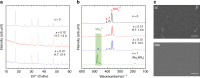
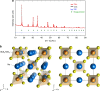
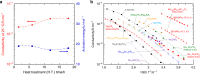
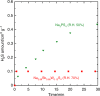
Solid electrolytes are key materials to enable solid-state rechargeable batteries, a promising technology that could address the safety and energy density issues. Here, we report a sulfide sodium-ion conductor, Na2.88Sb0.88W0.12S4, with conductivity superior to that of the benchmark electrolyte, Li10GeP2S12. Partial substitution of antimony in Na3SbS4 with tungsten introduces sodium vacancies and tetragonal to cubic phase transition, giving rise to the highest room-temperature conductivity of 32 mS cm-1 for a sintered body, Na2.88Sb0.88W0.12S4. Moreover, this sulfide possesses additional advantages including stability against humid atmosphere and densification at much lower sintering temperatures than those (>1000 °C) of typical oxide sodium-ion conductors. The discovery of the fast sodium-ion conductors boosts the ongoing research for solid-state rechargeable battery technology with high safety, cost-effectiveness, large energy and power densities.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Takada K. Progress and prospective of solid-state lithium batteries. Acta Mater. 2013;61:759–770. doi: 10.1016/j.actamat.2012.10.034. - DOI
-
- Manthiram A, Yu X, Wang S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017;2:16103. doi: 10.1038/natrevmats.2016.103. - DOI
-
- Janek J, Zeier WG. A solid future for battery development. Nat. Energy. 2016;1:1–4. doi: 10.1038/nenergy.2016.141. - DOI
-
- Kato Y, et al. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy. 2016;1:16030. doi: 10.1038/nenergy.2016.30. - DOI
-
- Hayashi A, Sakuda A, Tatsumisago M. Development of sulfide solid electrolytes and interface formation processes for bulk-type all-solid-state Li and Na batteries. Front. Energy Res. 2016;4:1–13. doi: 10.3389/fenrg.2016.00025. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

