Vasoactive Intestinal Peptide induces glucose and neutral amino acid uptake through mTOR signalling in human cytotrophoblast cells
- PMID: 31748639
- PMCID: PMC6868285
- DOI: 10.1038/s41598-019-53676-3
Vasoactive Intestinal Peptide induces glucose and neutral amino acid uptake through mTOR signalling in human cytotrophoblast cells
Abstract
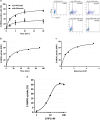
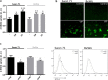
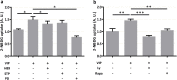
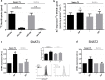
The transport of nutrients across the placenta involves trophoblast cell specific transporters modulated through the mammalian target of rapamycin (mTOR). The vasoactive intestinal peptide (VIP) has embryotrophic effects in mice and regulates human cytotrophoblast cell migration and invasion. Here we explored the effect of VIP on glucose and System A amino acid uptake by human trophoblast-derived cells (Swan 71 and BeWo cell lines). VIP activated D-glucose specific uptake in single cytotrophoblast cells in a concentration-dependent manner through PKA, MAPK, PI3K and mTOR signalling pathways. Glucose uptake was reduced in VIP-knocked down cytotrophoblast cells. Also, VIP stimulated System A amino acid uptake and the expression of GLUT1 glucose transporter and SNAT1 neutral amino acid transporter. VIP increased mTOR expression and mTOR/S6 phosphorylation whereas VIP silencing reduced mTOR mRNA and protein expression. Inhibition of mTOR signalling with rapamycin reduced the expression of endogenous VIP and of VIP-induced S6 phosphorylation. Our findings support a role of VIP in the transport of glucose and neutral amino acids in cytotrophoblast cells through mTOR-regulated pathways and they are instrumental for understanding the physiological regulation of nutrient sensing by endogenous VIP at the maternal-foetal interface.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Growth impairment, increased placental glucose uptake and altered transplacental transport in VIP deficient pregnancies: Maternal vs. placental contributions.Biochim Biophys Acta Mol Basis Dis. 2021 Oct 1;1867(10):166207. doi: 10.1016/j.bbadis.2021.166207. Epub 2021 Jun 26. Biochim Biophys Acta Mol Basis Dis. 2021. PMID: 34186168
-
Vasoactive intestinal peptide induces metabolic rewiring of human-derived cytotrophoblast cells to promote cell migration.Biochim Biophys Acta Mol Cell Res. 2025 Feb;1872(2):119886. doi: 10.1016/j.bbamcr.2024.119886. Epub 2024 Dec 7. Biochim Biophys Acta Mol Cell Res. 2025. PMID: 39653085
-
Trophoblast cells inhibit neutrophil extracellular trap formation and enhance apoptosis through vasoactive intestinal peptide-mediated pathways.Hum Reprod. 2017 Jan;32(1):55-64. doi: 10.1093/humrep/dew292. Epub 2016 Dec 7. Hum Reprod. 2017. PMID: 27932441
-
Placental regulation of fetal nutrient supply.Curr Opin Clin Nutr Metab Care. 2013 May;16(3):292-7. doi: 10.1097/MCO.0b013e32835e3674. Curr Opin Clin Nutr Metab Care. 2013. PMID: 23416721 Review.
-
Mechanistic Target of Rapamycin Is a Novel Molecular Mechanism Linking Folate Availability and Cell Function.J Nutr. 2017 Jul;147(7):1237-1242. doi: 10.3945/jn.117.248823. Epub 2017 Jun 7. J Nutr. 2017. PMID: 28592519 Free PMC article. Review.
Cited by
-
Neurotransmitters regulate β cells insulin secretion: A neglected factor.World J Clin Cases. 2023 Oct 6;11(28):6670-6679. doi: 10.12998/wjcc.v11.i28.6670. World J Clin Cases. 2023. PMID: 37901031 Free PMC article. Review.
-
The neuropeptide VIP potentiates intestinal innate type 2 and type 3 immunity in response to feeding.Mucosal Immunol. 2022 Apr;15(4):629-641. doi: 10.1038/s41385-022-00516-9. Epub 2022 May 2. Mucosal Immunol. 2022. PMID: 35501356
-
Fetomaternal Expression of Glucose Transporters (GLUTs)-Biochemical, Cellular and Clinical Aspects.Nutrients. 2022 May 12;14(10):2025. doi: 10.3390/nu14102025. Nutrients. 2022. PMID: 35631166 Free PMC article. Review.
References
-
- Mahendran D, et al. Na+ transport, H+ concentration gradient dissipation, and system A amino acid transporter activity in purified microvillous plasma membrane isolated from first-trimester human placenta: Comparison with the term microvillous membrane. Am. J. Obstet. Gynecol. 1994;171:1534–1540. doi: 10.1016/0002-9378(94)90397-2. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

