Bacterial siderophores in community and host interactions
- PMID: 31748738
- PMCID: PMC7116523
- DOI: 10.1038/s41579-019-0284-4
Bacterial siderophores in community and host interactions
Abstract
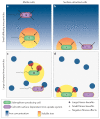
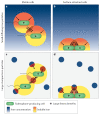
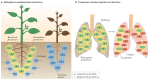
Iron is an essential trace element for most organisms. A common way for bacteria to acquire this nutrient is through the secretion of siderophores, which are secondary metabolites that scavenge iron from environmental stocks and deliver it to cells via specific receptors. While there has been tremendous interest in understanding the molecular basis of siderophore synthesis, uptake and regulation, questions about the ecological and evolutionary consequences of siderophore secretion have only recently received increasing attention. In this Review, we outline how eco-evolutionary questions can complement the mechanistic perspective and help to obtain a more integrated view of siderophores. In particular, we explain how secreted diffusible siderophores can affect other community members, leading to cooperative, exploitative and competitive interactions between individuals. These social interactions in turn can spur co-evolutionary arms races between strains and species, lead to ecological dependencies between them and potentially contribute to the formation of stable communities. In brief, this Review shows that siderophores are much more than just iron carriers: they are important mediators of interactions between members of microbial assemblies and the eukaryotic hosts they inhabit.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Andrews SC, Robinson AK, Rodriguez-Quinones F. Bacterial iron homeostasis. FEMS Microbiol Rev. 2003;27:215–237. - PubMed
-
- Boyd PW, Ellwood MJ. The biogeochemical cycle of iron in the ocean. Nat Geosci. 2010;3:675–682.
-
- Guerinot ML. Microbial iron transport. Annu Rev Microbiol. 1994;48:743–772. - PubMed
-
- Ratledge C, Dover LG. Iron metabolism in pathogenic bacteria. Annu Rev Microbiol. 2000;54:881–941. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

