A Specific Mutation in Muc2 Determines Early Dysbiosis in Colitis-Prone Winnie Mice
- PMID: 31748792
- PMCID: PMC7054774
- DOI: 10.1093/ibd/izz279
A Specific Mutation in Muc2 Determines Early Dysbiosis in Colitis-Prone Winnie Mice
Abstract
Background: Inflammatory bowel disease (IBD), including Crohn disease (CD) and ulcerative colitis (UC), is a multifactorial disorder characterized by chronic inflammation and altered gut barrier function. Dysbiosis, a condition defined by dysregulation of the gut microbiome, has been reported in patients with IBD and in experimental models of colitis. Although several factors have been implicated in directly affecting gut microbial composition, the genetic determinants impacting intestinal dysbiosis in IBD remain relatively unknown.
Methods: We compared the microbiome of normal, uninflamed wild-type (WT) mice with that of a murine model of UC (ie, Winnie strain). Winnie mice possess a missense mutation in Muc2 that manifests in altered mucus production as early as 4 weeks of age, with ensuing colonic inflammation. To better address the potential role of mutant Muc2 in promoting dysbiosis in Winnie mice, we evaluated homozygous mutant mice (Winnie-/-) with their WT littermates that, after weaning from common mothers, were caged separately according to genotype. Histologic and inflammatory status were assessed over time, along with changes in their respective microbiome compositions.
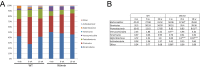
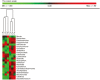
Results: Dysbiosis in Winnie mice was already established at 4 weeks of age, before histologic evidence of gut inflammatory changes, in which microbial communities diverged from that derived from their mothers. Furthermore, dysbiosis persisted until 12 weeks of age, with peak differences in microbiome composition observed between Winnie and WT mice at 8 weeks of age. The relative abundance of Bacteroidetes was greater in Winnie compared with WT mice. Verrucomicrobia was detected at the highest relative levels in 4-week-old Winnie mice; in particular, Akkermansia muciniphila was among the most abundant species found at 4 weeks of age.
Conclusions: Our results demonstrate that mutant genetic determinants involved in the complex regulation of intestinal homeostasis, such as that observed in Winnie mice, are able to promote early gut dysbiosis that is independent from maternal microbial transfer, including breastfeeding. Our data provide evidence for intestinal dysbiosis attributed to a Muc2-driven mucus defect that leads to colonic inflammation and may represent an important target for the design of future interventional studies.
Keywords: dysbiosis; inflammatory bowel disease (IBD); microbiome; murine model of ulcerative colitis (UC).
© 2019 Crohn’s & Colitis Foundation. Published by Oxford University Press on behalf of Crohn’s & Colitis Foundation.
Figures




References
-
- Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

