Liraglutide for perioperative management of hyperglycaemia in cardiac surgery patients: a multicentre randomized superiority trial
- PMID: 31749275
- PMCID: PMC7079116
- DOI: 10.1111/dom.13927
Liraglutide for perioperative management of hyperglycaemia in cardiac surgery patients: a multicentre randomized superiority trial
Abstract
Aims: Most cardiac surgery patients, with or without diabetes, develop perioperative hyperglycaemia, for which intravenous insulin is the only therapeutic option. This is labour-intensive and carries a risk of hypoglycaemia. We hypothesized that preoperative administration of the glucagon-like peptide-1 receptor agonist liraglutide reduces the number of patients requiring insulin for glycaemic control during cardiac surgery.
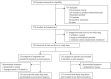
Materials and methods: In this randomized, blinded, placebo-controlled, parallel-group, balanced (1:1), multicentre randomized, superiority trial, adult patients undergoing cardiac surgery in four Dutch tertiary hospitals were randomized to receive 0.6 mg subcutaneous liraglutide on the evening before surgery and 1.2 mg after induction of anaesthesia or matching placebo. Blood glucose was measured hourly and controlled using an insulin-bolus algorithm. The primary outcome was insulin administration for blood glucose >8.0 mmol/L in the operating theatre. Research pharmacists used centralized, stratified, variable-block, randomization software. Patients, care providers and study personnel were blinded to treatment allocation.
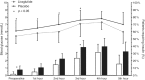
Results: Between June 2017 and August 2018, 278 patients were randomized to liraglutide (139) or placebo (139). All patients receiving at least one study drug injection were included in the intention-to-treat analyses (129 in the liraglutide group, 132 in the placebo group). In the liraglutide group, 55 (43%) patients required additional insulin compared with 80 (61%) in the placebo group and absolute difference 18% (95% confidence interval 5.9-30.0, P = 0.003). Dose and number of insulin injections and mean blood glucose were all significantly lower in the liraglutide group. We observed no difference in the incidence of hypoglycaemia, nausea and vomiting, mortality or postoperative complications.
Conclusions: Preoperative liraglutide, compared with placebo, reduces insulin requirements while improving perioperative glycaemic control during cardiac surgery.
© 2019 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
M.J.V., M.B.G., B.T., M.B.G., T.S. and M.G.W. have no conflicts to declare. A.H.H. and J.H. have received a grant from the European Society of Anaesthesiology. R.A.B. acts as a consultant for Philips Research. M.W.H. is Executive Section Editor Pharmacology with
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

