Pharmacological Inhibitors of the NLRP3 Inflammasome
- PMID: 31749805
- PMCID: PMC6842943
- DOI: 10.3389/fimmu.2019.02538
Pharmacological Inhibitors of the NLRP3 Inflammasome
Abstract
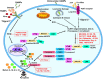
Inflammasomes play a crucial role in innate immunity by serving as signaling platforms which deal with a plethora of pathogenic products and cellular products associated with stress and damage. By far, the best studied and most characterized inflammasome is NLRP3 inflammasome, which consists of NLRP3 (nucleotide-binding domain leucine-rich repeat (NLR) and pyrin domain containing receptor 3), ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain), and procaspase-1. Activation of NLRP3 inflammasome is mediated by highly diverse stimuli. Upon activation, NLRP3 protein recruits the adapter ASC protein, which recruits the procaspase-1 resulting in its cleavage and activation, inducing the maturation, and secretion of inflammatory cytokines and pyroptosis. However, aberrant activation of the NLRP3 inflammasome is implicated in various diseases including diabetes, atherosclerosis, metabolic syndrome, cardiovascular, and neurodegenerative diseases; raising a tremendous clinical interest in exploring the potential inhibitors of NLRP3 inflammasome. Recent investigations have disclosed various inhibitors of the NLRP3 inflammasome pathway which were validated through in vitro studies and in vivo experiments in animal models of NLRP3-associated disorders. Some of these inhibitors directly target the NLRP3 protein whereas some are aimed at other components and products of the inflammasome. Direct targeting of NLRP3 protein can be a better choice because it can prevent off target immunosuppressive effects, thus restrain tissue destruction. This paper will review the various pharmacological inhibitors of the NLRP3 inflammasome and will also discuss their mechanism of action.
Keywords: IL-1β; MCC950; NLRP3 inflammasome; drug screening; inhibitors.
Copyright © 2019 Zahid, Li, Kombe, Jin and Tao.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

