Functional Characterization of the Grapevine γ-Glutamyl Transferase/Transpeptidase (E.C. 2.3.2.2) Gene Family Reveals a Single Functional Gene Whose Encoded Protein Product Is Not Located in Either the Vacuole or Apoplast
- PMID: 31749820
- PMCID: PMC6843540
- DOI: 10.3389/fpls.2019.01402
Functional Characterization of the Grapevine γ-Glutamyl Transferase/Transpeptidase (E.C. 2.3.2.2) Gene Family Reveals a Single Functional Gene Whose Encoded Protein Product Is Not Located in Either the Vacuole or Apoplast
Abstract
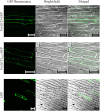
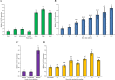
γ-glutamyl transferases/transpeptidases (E.C. 2.3.2.2, GGTs) are involved in the catabolism of many compounds that are conjugated to glutathione (GSH), which have a variety of roles. GSH can act as storage and transport vehicle for reduced sulfur; it is involved in the detoxification of xenobiotics and also acts as a redox buffer by utilizing its thiol residue to protect against reactive oxygen species, which accumulate in response to biotic and abiotic stress. Furthermore, many distinctive flavor and aroma compounds in Sauvignon blanc wines originate from odorless C5- and C6-GSH conjugates or their GGT catabolized derivatives. These precursors are then processed into their volatile forms by yeast during fermentation. In many plant species, two or more isoforms of GGTs exist that target GSH-conjugates to either the apoplast or the vacuole. A bioinformatics approach identified multiple GGT candidates in grapevine (Vitis vinifera). However, only a single candidate, VvGGT3, has all the conserved residues needed for GGT activity. This is intriguing given the variety of roles of GSH and GGTs in plant cells. Characterization of VvGGT3 from cv. Sauvignon blanc was then undertaken. The VvGGT3 transcript is present in roots, leaves, inflorescences, and tendril and at equal abundance in the skin, pulp, and seed of mature berries and shows steady accumulation over the course of whole berry development. In addition, the VvGGT3 transcript in whole berries is upregulated upon Botrytis cinerea infection as well as mechanical damage to leaf tissue. VvGGT3-GFP fusion proteins transiently over-expressed in onion cells were used to study subcellular localization. To confirm VvGGT3 activity and localization in vivo, the fluorescent γ-glutamyl-7-amido-4-methylcoumarin substrate was added to Nicotiana benthamiana leaves transiently over-expressing VvGGT3. In combination, these results suggest that the functional VvGGT3 is associated with membrane-like structures. This is not consistent with its closely related functionally characterized GGTs from Arabidopsis, radish and garlic.
Keywords: GGT; New Zealand Sauvignon blanc; glutathione; grape berry development; volatile thiol; γ-glutamyl transpeptidase.
Copyright © 2019 Philips, Dumin and Winefield.
Figures


Similar articles
-
Effects of irrigation on ecophysiology, sugar content andthiol precursors (3-S-cysteinylhexan-1-ol and 3-S-glutathionylhexan-1-ol) on Vitis vinifera cv. Sauvignon Blanc.Plant Physiol Biochem. 2021 Jul;164:247-259. doi: 10.1016/j.plaphy.2021.04.029. Epub 2021 May 7. Plant Physiol Biochem. 2021. PMID: 34015690
-
Tissue-specific mRNA expression profiling in grape berry tissues.BMC Genomics. 2007 Jun 21;8:187. doi: 10.1186/1471-2164-8-187. BMC Genomics. 2007. PMID: 17584945 Free PMC article.
-
Glutathione conjugates in the vacuole are degraded by gamma-glutamyl transpeptidase GGT3 in Arabidopsis.Plant J. 2007 Mar;49(5):878-88. doi: 10.1111/j.1365-313X.2006.03005.x. Plant J. 2007. PMID: 17316176
-
Bacterial Gamma-Glutamyl Transpeptidase, an Emerging Biocatalyst: Insights Into Structure-Function Relationship and Its Biotechnological Applications.Front Microbiol. 2021 Apr 9;12:641251. doi: 10.3389/fmicb.2021.641251. eCollection 2021. Front Microbiol. 2021. PMID: 33897647 Free PMC article. Review.
-
Degradation of glutathione and glutathione conjugates in plants.J Exp Bot. 2023 Jun 6;74(11):3313-3327. doi: 10.1093/jxb/erad018. J Exp Bot. 2023. PMID: 36651789 Review.
Cited by
-
γ-Glutamylcysteine Exerts Neuroprotection Effects against Cerebral Ischemia/Reperfusion Injury through Inhibiting Lipid Peroxidation and Ferroptosis.Antioxidants (Basel). 2022 Aug 25;11(9):1653. doi: 10.3390/antiox11091653. Antioxidants (Basel). 2022. PMID: 36139727 Free PMC article.
-
Overlooked and misunderstood: can glutathione conjugates be clues to understanding plant glutathione transferases?Philos Trans R Soc Lond B Biol Sci. 2024 Nov 18;379(1914):20230365. doi: 10.1098/rstb.2023.0365. Epub 2024 Sep 30. Philos Trans R Soc Lond B Biol Sci. 2024. PMID: 39343017 Free PMC article. Review.
References
-
- Balakrishna S., Prabhune A. A. (2014). Gamma-glutamyl transferases: a structural, mechanistic and physiological perspective. Front. Biol. 9 (1), 51–65. 10.1007/s11515-014-1288-0 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

