Interleukin-1α deficiency reduces adiposity, glucose intolerance and hepatic de-novo lipogenesis in diet-induced obese mice
- PMID: 31749969
- PMCID: PMC6827792
- DOI: 10.1136/bmjdrc-2019-000650
Interleukin-1α deficiency reduces adiposity, glucose intolerance and hepatic de-novo lipogenesis in diet-induced obese mice
Abstract
Objective: While extensive research revealed that interleukin (IL)-1β contributes to insulin resistance (IR) development, the role of IL-1α in obesity and IR was scarcely studied. Using control, whole body IL-1α knockout (KO) or myeloid-cell-specific IL-1α-deficient mice, we tested the hypothesis that IL-1α deficiency would protect against high-fat diet (HFD)-induced obesity and its metabolic consequences.
Research design and methods: To induce obesity and IR, control and IL-1α KO mice were given either chow or HFD for 16 weeks. Glucose tolerance test was performed at 10 and 15 weeks, representing early and progressive stages of glucose intolerance, respectively. Liver and epididymal white adipose tissue (eWAT) samples were analyzed for general morphology and adipocyte size. Plasma levels of adiponectin, insulin, total cholesterol and triglyceride (TG), lipoprotein profile as well as hepatic lipids were analyzed. Expression of lipid and inflammation-related genes in liver and eWAT was analyzed. Primary mouse hepatocytes isolated from control mice were treated either with dimethyl sulfoxide (DMSO) (control) or 20 ng/mL recombinant IL-1α for 24 hours and subjected to gene expression analysis.
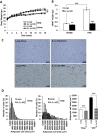
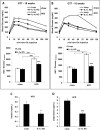
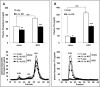
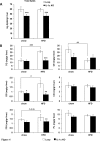
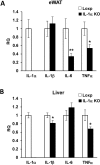
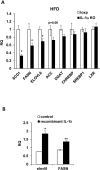
Results: Although total body weight gain was similar, IL-1α KO mice showed reduced adiposity and were completely protected from HFD-induced glucose intolerance. In addition, plasma total cholesterol and TG levels were lower and HFD-induced accumulation of liver TGs was completely inhibited in IL-1α KO compared with control mice. Expression of stearoyl-CoA desaturase1 (SCD1), fatty acid synthase (FASN), elongation of long-chain fatty acids family member 6 (ELOVL6), acetyl-CoA carboxylase (ACC), key enzymes that promote de-novo lipogenesis, was lower in livers of IL-1α KO mice. Treatment with recombinant IL-1α elevated the expression of ELOVL6 and FASN in mouse primary hepatocytes. Finally, mice with myeloid-cell-specific deletion of IL-1α did not show reduced adiposity and improved glucose tolerance.
Conclusions: We demonstrate a novel role of IL-1α in promoting adiposity, obesity-induced glucose intolerance and liver TG accumulation and suggest that IL-1α blockade could be used for treatment of obesity and its metabolic consequences.
Keywords: de novo lipogenesis; glucose intolerance; interleukin-1; obesity.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Hepatic stearoyl CoA desaturase 1 deficiency increases glucose uptake in adipose tissue partially through the PGC-1α-FGF21 axis in mice.J Biol Chem. 2019 Dec 20;294(51):19475-19485. doi: 10.1074/jbc.RA119.009868. Epub 2019 Nov 5. J Biol Chem. 2019. PMID: 31690632 Free PMC article.
-
Long-term exposure to a high-fat diet results in the development of glucose intolerance and insulin resistance in interleukin-1 receptor I-deficient mice.Am J Physiol Endocrinol Metab. 2013 Oct 1;305(7):E834-44. doi: 10.1152/ajpendo.00297.2013. Epub 2013 Aug 6. Am J Physiol Endocrinol Metab. 2013. PMID: 23921145 Free PMC article.
-
Proteoglycan 4 deficiency protects against glucose intolerance and fatty liver disease in diet-induced obese mice.Biochim Biophys Acta Mol Basis Dis. 2019 Feb 1;1865(2):494-501. doi: 10.1016/j.bbadis.2018.11.009. Epub 2018 Nov 15. Biochim Biophys Acta Mol Basis Dis. 2019. PMID: 30448542
-
Genetic control of de novo lipogenesis: role in diet-induced obesity.Crit Rev Biochem Mol Biol. 2010 Jun;45(3):199-214. doi: 10.3109/10409231003667500. Crit Rev Biochem Mol Biol. 2010. PMID: 20218765 Free PMC article. Review.
-
Pathway-selective insulin resistance and metabolic disease: the importance of nutrient flux.J Biol Chem. 2014 Jul 25;289(30):20462-9. doi: 10.1074/jbc.R114.576355. J Biol Chem. 2014. PMID: 24907277 Free PMC article. Review.
Cited by
-
Sex-Specific Effect of a High-Energy Diet on Body Composition, Gut Microbiota, and Inflammatory Markers in Rats.Nutrients. 2025 Mar 26;17(7):1147. doi: 10.3390/nu17071147. Nutrients. 2025. PMID: 40218905 Free PMC article.
-
The Effect of Tff3 Deficiency on the Liver of Mice Exposed to a High-Fat Diet.Biomedicines. 2025 Apr 23;13(5):1024. doi: 10.3390/biomedicines13051024. Biomedicines. 2025. PMID: 40426854 Free PMC article.
-
IL-1β promotes adipogenesis by directly targeting adipocyte precursors.Nat Commun. 2024 Sep 11;15(1):7957. doi: 10.1038/s41467-024-51938-x. Nat Commun. 2024. PMID: 39261467 Free PMC article.
-
Innate-Immunity Genes in Obesity.J Pers Med. 2021 Nov 14;11(11):1201. doi: 10.3390/jpm11111201. J Pers Med. 2021. PMID: 34834553 Free PMC article. Review.
-
Adipokines: masterminds of metabolic inflammation.Nat Rev Immunol. 2025 Apr;25(4):250-265. doi: 10.1038/s41577-024-01103-8. Epub 2024 Nov 7. Nat Rev Immunol. 2025. PMID: 39511425 Review.
References
-
- Miranda PJ, DeFronzo RA, Califf RM, et al. . Pathophysiology, and mechanisms. Am Heart J 2005;149:33–45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
