Eosinophil cytolysis on Immunoglobulin G is associated with microtubule formation and suppression of rho-associated protein kinase signalling
- PMID: 31750580
- PMCID: PMC6994386
- DOI: 10.1111/cea.13538
Eosinophil cytolysis on Immunoglobulin G is associated with microtubule formation and suppression of rho-associated protein kinase signalling
Abstract
Background: The presence of eosinophils in the airway is associated with asthma severity and risk of exacerbations. Cell-free eosinophil granules are found in tissues in eosinophilic diseases, including asthma. This suggests that eosinophils have lysed and released cellular content, likely harming tissues.
Objective: The present study explores the mechanism of CD32- and αMß2 integrin-dependent eosinophil cytolysis of IL3-primed blood eosinophils seeded on heat-aggregated immunoglobulin G (HA-IgG).
Methods: Cytoskeletal events and signalling pathways potentially involved in cytolysis were assessed using inhibitors. The level of activation of the identified events and pathways involved in cytolysis was measured. In addition, the links between these identified pathways and changes in degranulation (exocytosis) and adhesion were analysed.
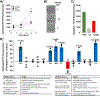
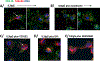
Results: Cytolysis of IL3-primed eosinophils was dependent on the production of reactive oxygen species (ROS) and downstream phosphorylation of p-38 MAPK. In addition, formation of microtubule (MT) arrays was necessary for cytolysis and was accompanied by changes in MT dynamics as measured by phosphorylation status of stathmin and microtubule-associated protein 4 (MAP4), the latter of which was regulated by ROS production. Reduced ROCK signalling preceded cytolysis, which was associated with eosinophil adhesion and reduced migration.
Conclusion and clinical relevance: In this CD32- and αMß2 integrin-dependent adhesion model, lysing eosinophils exhibit reduced migration and ROCK signalling, as well as both MT dynamic changes and p-38 phosphorylation downstream of ROS production. We propose that interfering with these pathways would modulate eosinophil cytolysis and subsequent eosinophil-driven tissue damage.
Keywords: Eosinophil; IL3; adhesion; cytolysis; degranulation; immunoglobulin G; microtubule; priming.
© 2019 John Wiley & Sons Ltd.
Figures


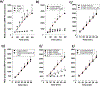

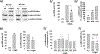
References
-
- Busse W, Chupp G, Nagase H, Albers FC, Doyle S, Shen Q, Bratton DJ, Gunsoy NB, Anti-IL-5 treatments in patients with severe asthma by blood eosinophil thresholds: Indirect treatment comparison. J Allergy Clin Immunol 2019;143: 190–200 e20. - PubMed
-
- Yasruel Z, Humbert M, Kotsimbos TC, Ploysongsang Y, Minshall E, Durham SR, Pfister R, Menz G, Tavernier J, Kay AB, Hamid Q, Membrane-bound and soluble alpha IL-5 receptor mRNA in the bronchial mucosa of atopic and nonatopic asthmatics. Am J Respir Crit Care Med 1997;155: 1413–18. - PubMed
-
- Miyajima A, Mui AL, Ogorochi T, Sakamaki K, Receptors for granulocyte macrophage colony stimulating factor, interleukin-3, and interleukin-5. Blood 1993;82: 1960–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

