Follow-up strategies following completion of primary cancer treatment in adult cancer survivors
- PMID: 31750936
- PMCID: PMC6870787
- DOI: 10.1002/14651858.CD012425.pub2
Follow-up strategies following completion of primary cancer treatment in adult cancer survivors
Abstract
Background: Most cancer survivors receive follow-up care after completion of treatment with the primary aim of detecting recurrence. Traditional follow-up consisting of fixed visits to a cancer specialist for examinations and tests are expensive and may be burdensome for the patient. Follow-up strategies involving non-specialist care providers, different intensity of procedures, or addition of survivorship care packages have been developed and tested, however their effectiveness remains unclear.
Objectives: The objective of this review is to compare the effect of different follow-up strategies in adult cancer survivors, following completion of primary cancer treatment, on the primary outcomes of overall survival and time to detection of recurrence. Secondary outcomes are health-related quality of life, anxiety (including fear of recurrence), depression and cost.
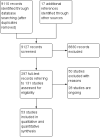
Search methods: We searched CENTRAL, MEDLINE, Embase, four other databases and two trials registries on 11 December 2018 together with reference checking, citation searching and contact with study authors to identify additional studies.
Selection criteria: We included all randomised trials comparing different follow-up strategies for adult cancer survivors following completion of curatively-intended primary cancer treatment, which included at least one of the outcomes listed above. We compared the effectiveness of: 1) non-specialist-led follow-up (i.e. general practitioner (GP)-led, nurse-led, patient-initiated or shared care) versus specialist-led follow-up; 2) less intensive versus more intensive follow-up (based on clinical visits, examinations and diagnostic procedures) and 3) follow-up integrating additional care components relevant for detection of recurrence (e.g. patient symptom education or monitoring, or survivorship care plans) versus usual care.
Data collection and analysis: We used the standard methodological guidelines by Cochrane and Cochrane Effective Practice and Organisation of Care (EPOC). We assessed the certainty of the evidence using the GRADE approach. For each comparison, we present synthesised findings for overall survival and time to detection of recurrence as hazard ratios (HR) and for health-related quality of life, anxiety and depression as mean differences (MD), with 95% confidence intervals (CI). When meta-analysis was not possible, we reported the results from individual studies. For survival and recurrence, we used meta-regression analysis where possible to investigate whether the effects varied with regards to cancer site, publication year and study quality.
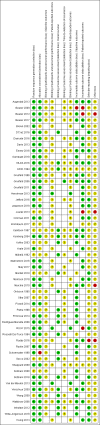
Main results: We included 53 trials involving 20,832 participants across 12 cancer sites and 15 countries, mainly in Europe, North America and Australia. All the studies were carried out in either a hospital or general practice setting. Seventeen studies compared non-specialist-led follow-up with specialist-led follow-up, 24 studies compared intensity of follow-up and 12 studies compared patient symptom education or monitoring, or survivorship care plans with usual care. Risk of bias was generally low or unclear in most of the studies, with a higher risk of bias in the smaller trials. Non-specialist-led follow-up compared with specialist-led follow-up It is uncertain how this strategy affects overall survival (HR 1.21, 95% CI 0.68 to 2.15; 2 studies; 603 participants), time to detection of recurrence (4 studies, 1691 participants) or cost (8 studies, 1756 participants) because the certainty of the evidence is very low. Non-specialist- versus specialist-led follow up may make little or no difference to health-related quality of life at 12 months (MD 1.06, 95% CI -1.83 to 3.95; 4 studies; 605 participants; low-certainty evidence); and probably makes little or no difference to anxiety at 12 months (MD -0.03, 95% CI -0.73 to 0.67; 5 studies; 1266 participants; moderate-certainty evidence). We are more certain that it has little or no effect on depression at 12 months (MD 0.03, 95% CI -0.35 to 0.42; 5 studies; 1266 participants; high-certainty evidence). Less intensive follow-up compared with more intensive follow-up Less intensive versus more intensive follow-up may make little or no difference to overall survival (HR 1.05, 95% CI 0.96 to 1.14; 13 studies; 10,726 participants; low-certainty evidence) and probably increases time to detection of recurrence (HR 0.85, 95% CI 0.79 to 0.92; 12 studies; 11,276 participants; moderate-certainty evidence). Meta-regression analysis showed little or no difference in the intervention effects by cancer site, publication year or study quality. It is uncertain whether this strategy has an effect on health-related quality of life (3 studies, 2742 participants), anxiety (1 study, 180 participants) or cost (6 studies, 1412 participants) because the certainty of evidence is very low. None of the studies reported on depression. Follow-up strategies integrating additional patient symptom education or monitoring, or survivorship care plans compared with usual care: None of the studies reported on overall survival or time to detection of recurrence. It is uncertain whether this strategy makes a difference to health-related quality of life (12 studies, 2846 participants), anxiety (1 study, 470 participants), depression (8 studies, 2351 participants) or cost (1 studies, 408 participants), as the certainty of evidence is very low.
Authors' conclusions: Evidence regarding the effectiveness of the different follow-up strategies varies substantially. Less intensive follow-up may make little or no difference to overall survival but probably delays detection of recurrence. However, as we did not analyse the two outcomes together, we cannot make direct conclusions about the effect of interventions on survival after detection of recurrence. The effects of non-specialist-led follow-up on survival and detection of recurrence, and how intensity of follow-up affects health-related quality of life, anxiety and depression, are uncertain. There was little evidence for the effects of follow-up integrating additional patient symptom education/monitoring and survivorship care plans.
Copyright © 2019 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Beverley Lim Høeg (BLH), none known
Pernille Envold Bidstrup (PEB), none known
Randi Valbjørn Karlsen (RVK), none known
Anne Sofie Friberg (ASF), none known
Vanna Albieri (VA), none known
Susanne Oksbjerg Dalton, none known
Lena Saltbæk (LS), none known
Klaus Kaae Andersen (KKA), after completing the work on the review and near to its final publication, KKA started working at Astra Zeneca.
Trine Allerslev Horsboel (TAH), none known
Christoffer Johansen (CJ), none known
Figures















Update of
- doi: 10.1002/14651858.CD012425
References
References to studies included in this review
Augestad 2013 {published data only}
-
- Augestad KM, Norum J, Dehof S, Aspevik R, Ringberg U, Nestvold T, et al. Cost‐effectiveness and quality of life in surgeon versus general practitioner‐organised colon cancer surveillance: a randomised controlled trial. BMJ Open 2013;3(4):e002391. [DOI: 10.1136/bmjopen-2012-002391] - DOI - PMC - PubMed
-
- Augestad KM, Vonen B, Aspevik R, Nestvold T, Ringberg U, Johnsen R, et al. Should the surgeon or the general practitioner (GP) follow up patients after surgery for colon cancer? A randomized controlled trial protocol focusing on quality of life, cost‐effectiveness and serious clinical events. BMC Health Services Research 2008;8:137. [DOI: 10.1186/1472-6963-8-137] - DOI - PMC - PubMed
-
- NCT00572143. Follow‐up after surgery for colon cancer. General practice vs. surgical‐based follow‐up?. clinicaltrials.gov/ct2/show/NCT00572143 (first received 20 December 2016).
Beaver 2009 {published data only}
-
- Beaver K, Hollingworth W, McDonald R, Dunn G, Tysver‐Robinson D, Thomson L, et al. Is telephone follow‐up by specialist nurses a cost effective approach?. European Journal of Cancer Supplements 2009;7(2‐3):230‐1.
Beaver 2012 {published data only}
Beaver 2017 {published data only}
-
- Beaver K, Williamson S, Martin‐Hirsch P, Keating PJ, Tomlinson A, Sutton C, et al. ENDCAT: endometrial cancer telephone follow‐up trial. Psycho‐oncology 2013;22:19.
-
- Beaver K, Williamson S, Sutton C, Hollingworth W, Gardner A, Allton B, et al. Comparing hospital and telephone follow‐up for patients treated for stage‐I endometrial cancer (ENDCAT trial): a randomised, multicentre, non‐inferiority trial. BJOG 2017;124:150‐60. [DOI: ] - PubMed
-
- Beaver K, Williamson S, Sutton C, Hollingworth W, Gardner A, Tomlinson A, et al. Comparing hospital and telephone follow‐up for women treated for endometrial cancer (ENDCAT trial). Psycho‐oncology 2016;25:6‐7. - PubMed
-
- Williamson S, Beaver K, Gardner A, Martin‐Hirsch P. Telephone follow‐up after treatment for endometrial cancer: a qualitative study of patients' and clinical nurse specialists' experiences in the ENDCAT trial. European Journal of Oncology Nursing 2018;34:61‐7. - PubMed
Brown 2002 {published data only}
-
- Brown L, Payne S, Royle G. Patient‐initiated follow‐up of breast cancer. Psycho‐oncology 2002;11(4):346‐55. - PubMed
D'Cruz 2016 {published data only}
-
- D'Cruz A, Vaish R, Gupta S, Arya S, Hawaldar RW, Shah S. Does addition of neck ultrasonography to physical examination, in follow‐up of patients with early stage, clinically node negative oral cancers, influence outcome? A randomized control trial (RCT). Journal of Clinical Oncology 2016;34(15 Suppl 6):no pagination.
-
- D'Cruz AK, Vaish R, Kapre N, Dandekar M, Gupta S, Hawaldar R, et al. Elective versus therapeutic neck dissection in node‐negative oral cancer. New England Journal of Medicine 2015;373(6):521‐9. - PubMed
-
- NCT00193765. Elective vs therapeutic neck dissection in treatment of early node negative squamous carcinoma of oral cavity. clinicaltrials.gov/ct2/show/NCT00193765 (first received 20 December 2016).
Damude 2016 {published data only}
-
- Damude S, Hoekstra‐Weebers JE, Francken AB, Ter Meulen S, Bastiaannet E, Hoekstra HJ. Prospective randomized clinical trial for the evaluation of a stage adjusted reduced follow‐up schedule in cutaneous melanoma patients: results after one year. Annals of Surgical Oncology 2016;23(1 Suppl 1):S30. - PMC - PubMed
-
- Damude S, Hoekstra‐Weebers JE, Francken AB, Ter Meulen S, Bastiaannet E, Hoekstra HJ. The MELFO‐study: prospective randomized clinical trial for the evaluation of a stage‐adjusted reduced follow‐up schedule in cutaneous melanoma patients ‐ results after 1 year. Annals of Surgical Oncology 2016;23(9):2762‐71. [DOI: 10.1245/s10434-016-5263-7] - DOI - PMC - PubMed
-
- Deckers E, Hoekstra‐Weebers J, Damude S, Francken A, Ter Meulen S, Bastiaannet E, et al. The MELFO‐study: a multi‐center prospective randomized clinical trial on the effects of a reduced stage‐adjusted follow‐up schedule on cutaneous melanoma IB‐IIC patients: results after 3‐years. Annals of Surgical Oncology 2018;25(1 Suppl 1):S40. - PMC - PubMed
-
- NCT01018004. Comparing follow‐up schedules in patients with newly diagnosed stage IB or stage II melanoma. clinicaltrials.gov/ct2/show/NCT01018004 (first received 20 December 2016).
Davis 2013 {published data only}
-
- Davis KM, Dawson D, Kelly S, Red S, Penek S, Lynch J, et al. Monitoring of health‐related quality of life and symptoms in prostate cancer survivors: a randomized trial. Journal of Supportive Oncology 2013;11(4):174‐82. - PubMed
Emery 2016 {published data only}
-
- ACTRN12610000938000. The ProCare Trial: a randomised controlled trial of follow up of men with prostate cancer in primary care. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=336116 (first received 20 December 2016).
-
- Emery J, Schofield P, Jefford M, King M, Pirotta M, Hayne D, et al. The ProCare trial: a phase II randomised controlled trial of shared care for follow‐up of men with prostate cancer. Asia‐Pacific Journal of Clinical Oncology 2014;10:157.
-
- King M. Procare/PC4 study update. Asia‐Pacific Journal of Clinical Oncology 2012;8:23.
Gambazzi 2018 {published data only}
GILDA 2016 {published data only}
-
- GILDA (Gruppo Italiano di Lavoro per la Diagnosi Anticipata). A randomized trial of intensive versus minimalist follow‐up of patients with resected Dukes B‐C colorectal cancer: the pilot phase. Tumori 1998;84(Suppl):89.
-
- Grossmann EM, Johnson FE, Virgo KS, Longo WE, Fossati R. Follow‐up of colorectal cancer patients after resection with curative intent‐the GILDA trial. Surgical Oncology 2004;13(2‐3):119‐24. - PubMed
-
- NCT02409472. Intensive versus minimal surveillance of patients with resected Dukes B2‐C colorectal carcinoma. clinicaltrials.gov/ct2/show/NCT02409472 (first received 20 December 2016).
GIVIO 1994 {published data only}
-
- Coleman EA. Commentary on impact of follow‐up testing on survival and health‐related quality of life in breast cancer patients: a multicenter randomized controlled trial [original article appears in JAMA 1994;271(20):1587‐92]. ONS Nursing Scan in Oncology 1994; Vol. 3, issue 5:8‐8. - PubMed
-
- GIVIO Investigators. Impact of follow‐up testing on survival and health‐related quality of life in breast cancer patients: a multicenter randomized controlled trial. JAMA 1994;271(20):1587‐92. - PubMed
-
- Liberati A. The GIVIO trial on the impact of follow‐up care on survival and quality of life in breast cancer patients. Interdisciplinary Group for Cancer Care Evaluation. Annals of Oncology 1995;6(Suppl 2):41‐6. - PubMed
Grunfeld 1996 {published data only}
-
- Goodwin PJ. Women with breast cancer were more satisfied with general practitioner care than with outpatient clinic care. Commentary on Grunfeld E, Fitzpatrick R, Mant D et al Comparison of breast cancer patient satisfaction with follow‐up in primary care versus specialist care: results from a randomized controlled trial BR J GEN PRACT 1999 Sep;49:705‐10. ACP Journal Club 2000;132(3):106. - PMC - PubMed
-
- Grunfeld E, Fitzpatrick R, Mant D, Yudkin P, Adewuyi‐Dalton R, Stewart J, et al. Comparison of breast cancer patient satisfaction with follow‐up in primary care versus specialist care: results from a randomized controlled trial. British Journal of General Practice 1999;49(446):705‐10. - PMC - PubMed
-
- Grunfeld E, Mant D, Vessey MP, Yudkin P. Evaluating primary care follow‐up of breast cancer: methods and preliminary results of three studies. Annals of Oncology 1995;6(Suppl 2):47‐52. - PubMed
Grunfeld 2006 {published and unpublished data}
-
- Grunfeld E, Julian J, Levine M, Pritchard K. A randomized controlled trial (RCT) of long‐term follow‐up for early stage breast cancer comparing family physician to specialist care: a report of secondary outcomes. Journal of Clinical Oncology 2006;24(18 Suppl):301s. - PubMed
-
- Grunfeld E, Levine M, Julian J, Pritchard K, Coyle D, Mirsky D. A randomized controlled trial (RCT) of routine follow‐up for early stage breast cancer: a comparison of primary care versus specialist care. Journal of Clinical Oncology 2004;22(14):665. - PubMed
-
- Grunfeld E, Levine M, Julian J, Pritchard K, Coyle D, Mirsky D. A randomized controlled trial (RCT) of routine follow‐up for early stage breast cancer: a comparison of primary care versus specialist care. Proceedings of the American Society of Clinical Oncology. 2004; Vol. 22:13 Suppl. - PubMed
-
- Grunfeld E, Levine MN, Julian JA, Coyle D, Szechtman B, Mirsky D, et al. Randomized trial of long‐term follow‐up for early‐stage breast cancer: a comparison of family physician versus specialist care. Journal of Clinical Oncology 2006;24(6):848‐55. - PubMed
-
- NCT00156039. Randomized trial of follow‐up strategies in breast cancer. clinicaltrials.gov/ct2/show/NCT00156039 (first received 20 December 2016).
Grunfeld 2011 {published and unpublished data}
-
- Boekhout AH, Maunsell E, Pond GR, Julian JA, Coyle D, Levine MN, et al. A survivorship care plan for breast cancer survivors: extended results of a randomized clinical trial. Journal of Cancer Survivorship 2015;9(4):683‐91. - PubMed
-
- Coyle D, Grunfeld E, Coyle K, Julian JA, Pond GR, Folkes A, et al. Cost‐effectiveness of a survivorship care plan for breast cancer survivors. Journal of Clinical Oncology 2011;29(15 Suppl 1):6082. - PubMed
-
- Coyle D, Grunfeld E, Coyle K, Pond G, Julian JA, Levine MN. Cost effectiveness of a survivorship care plan for breast cancer survivors. Journal of Oncology Practice 2014;10(2):e86‐92. - PubMed
-
- Grunfeld E, Julian JA, Pond G, Maunsell E, Coyle D, Folkes A, et al. Evaluating survivorship care plans: results of a randomized, clinical trial of patients with breast cancer. Journal of Clinical Oncology 2011;29(36):4755‐62. - PubMed
-
- Grunfeld E, Levine MN, Julian JA, Pond GR, Maunsell E, Folkes A, et al. Results of a multicenter randomized trial to evaluate a survivorship care plan for breast cancer survivors. Journal of Clinical Oncology 2011;29(15 Suppl 1):9005.
Hershman 2013 {published data only}
-
- Hershman DL, Greenlee H, Awad D, Kalinsky K, Maurer M, Kranwinkel G, et al. Randomized controlled trial of a clinic‐based survivorship intervention following adjuvant therapy in breast cancer survivors. Breast Cancer Research and Treatment 2013;138(3):795‐806. - PubMed
Jefford 2016 {published data only}
-
- Jefford M, Gough K, Drosdowsky A, Russell L, Aranda S, Butow PN, et al. A randomized controlled trial (RCT) of a nurse‐led supportive care package (survivor care) for survivors of colorectal cancer. Cancer Nursing 2015;38(4 Suppl 1):S16‐7.
-
- Jefford M, Gough K, Drosdowsky A, Russell L, Aranda S, Butow PN, et al. A randomized controlled trial (RCT) of a supportive care package (Survivor‐Care, SC) for survivors of colorectal cancer (CRC). Journal of Clinical Oncology 2015;33(15 Suppl 1):9566.
-
- Jefford M, Gough K, Drosdowsky A, Russell L, Aranda S, Butow PN, et al. A randomized controlled trial (RCT) of a supportive care package (SurvivorCare, SC) for survivors of colorectal cancer (CRC). Journal of Clinical Oncology 2016;34(3 Suppl 1):no pagination.
Jeppesen 2018 {published data only}
-
- Jeppesen MM, Jensen PT, Gilsa D, DePont Christensen R, Jensen MM, Mogensen O. The effect of patient‐initiated follow‐up on fear of recurrence and health care use: a randomized trial in early‐stage endometrial cancer. International Journal of Gynecological Cancer 2017;27(Supplement 4):1107.
-
- Jeppesen MM, Jensen PT, Hansen DG, Christensen RD, Mogensen O. Authors' reply re: Patient‐initiated follow‐up affects fear of recurrence and health care use: a randomised trial in early‐stage endometrial cancer. BJOG 2018;125(13):1778‐9. - PubMed
-
- Jeppesen MM, Jensen PT, Hansen DG, Christensen RD, Mogensen O. Patient‐initiated follow up affects fear of recurrence and healthcare use: a randomised trial in early‐stage endometrial cancer. BJOG 2018;125(13):1705‐14. - PubMed
-
- Tegnerowicz J. Re: Patient‐initiated follow up affects fear of recurrence and healthcare use: a randomised trial in early‐stage endometrial cancer. BJOG 2018;1125(13):1778. - PubMed
Juarez 2013 {published data only}
-
- Juarez G, Hurria A, Ferrell B. Effectiveness of an education intervention on quality of life in Latina breast cancer survivors. Psycho‐oncology 2012;21:110.
Kimman 2011 {published data only}
-
- Kimman ML, Dirksen CD, Falger P, Voogd A, Kessels A, Gijsen B, et al. Results of an RCT investigating the cost‐effectiveness of four follow‐up strategies after breast cancer. European Journal of Cancer (Suppl) 2009;7(2‐3):18.
-
- Kimman ML, Dirksen CD, Voogd AC, Falger P, Gijsen BC, Thuring M, et al. Economic evaluation of four follow‐up strategies after curative treatment for breast cancer: results of an RCT. European Journal of Cancer 2011;47(8):1175‐85. - PubMed
-
- Kimman ML, Dirksen CD, Voogd AC, Falger P, Gijsen BC, Thuring M, et al. Nurse‐led telephone follow‐up and an educational group programme after breast cancer treatment: results of a 2 x 2 randomised controlled trial. European Journal of Cancer 2011;47(7):1027‐36. - PubMed
Kirshbaum 2017 {published data only}
-
- Dent J, Topping A, Ferguson C, Stephenson J, McCoy M, Allinson V, et al. To follow up or not? A new model of supportive care for early breast cancer. Journal of Clinical Oncology 2011;29(15 Suppl 1):no pagination.
Kjeldsen 1997 {published data only}
-
- Kjeldsen BJ, Kronborg O, Fenger C, Jørgensen OD. A prospective randomized study of follow‐up after radical surgery for colorectal cancer. British Journal of Surgery 1997;84(5):666‐9. - PubMed
-
- Kjeldsen BJ, Kronborg O, Fenger C, Jørgensen OD. The pattern of recurrent colorectal cancer in a prospective randomised study and the characteristics of diagnostic tests. International Journal of Colorectal Disease 1997;12(6):329‐34. - PubMed
-
- Kjeldsen BJ, Thorsen H, Whalley D, Kronborg O. Influence of follow‐up on health‐related quality of life after radical surgery for colorectal cancer. Scandinavian Journal of Gastroenterology 1999;34(5):509‐15. - PubMed
-
- Kronborg O, Fenger C, Deichgräber E, Hansen L. Follow‐up after radical surgery for colorectal cancer. Design of a randomized study. Scandinavian Journal of Gastroenterology 1988;149:159‐62. - PubMed
Koinberg 2004 {published data only}
-
- Koinberg I, Engholm GB, Genell A, Holmberg L. A health economic evaluation of follow‐up after breast cancer surgery: results of an RCT study. Acta Oncologica 2009;48(1):99‐104. - PubMed
-
- Koinberg IL, Fridlund B, Engholm GB, Holmberg L. Comparison between nurse‐led check‐ups on demand and follow‐ups by a physician after breast cancer surgery. European Journal of Cancer 2003;1:S362. - PubMed
-
- Koinberg IL, Fridlund B, Engholm GB, Holmberg L. Nurse‐led follow‐up on demand or by a physician after breast cancer surgery: a randomised study. European Journal of Oncology Nursing 2004;8(2):109‐17; discussion 118‐20. - PubMed
Kokko 2003 {published data only}
-
- Kokko R, Hakama M, Holli K. Follow‐up cost of breast cancer patients with localized disease after primary treatment: a randomized trial. Breast Cancer Research and Treatment 2005;93(3):255‐60. - PubMed
-
- Kokko R, Hakama M, Holli K. Role of chest X‐ray in diagnosis of the first breast cancer relapse: a randomized trial. Breast Cancer Research and Treatment 2003;81(1):33‐9. - PubMed
Kvale 2016 {published data only}
-
- Kvale EA, Demark‐Wahnefried W, Meneses K, Bae S, Huang CH, Azuero CB, et al. Patient centered support in the survivorship care transition: outcomes from the patient‐owned survivorship care plan intervention. Cancer Research 2015;75(9 Suppl 1):no pagination. - PubMed
-
- Kvale EA, Huang CS, Meneses KM, Demark‐Wahnefried W, Bae S, Azuero CB, et al. Patient‐centered support in the survivorship care transition: outcomes from the patient‐owned survivorship care plan intervention. Cancer 2016;122(20):3232‐42. - PubMed
Mäkelä 1992 {published data only}
-
- Mäkelä J, Laitinen S, Kairaluoma MI. Early results of follow‐up after radical resection for colorectal cancer. Preliminary results of a prospective randomized trial. Surgical Oncology 1992;1(2):157‐61. - PubMed
-
- Mäkelä JT, Laitinen SO, Kairaluoma MI. Five‐year follow‐up after radical surgery for colorectal cancer. Results of a prospective randomized trial. Archives of Surgery 1995;130(10):1062‐7. - PubMed
Malmstrom 2016 {published data only}
-
- Malmström M, Ivarsson B, Klefsgård R, Persson K, Jakobsson U, Johansson J. The effect of a nurse led telephone supportive care programme on patients' quality of life, received information and health care contacts after oesophageal cancer surgery‐a six month RCT‐follow‐up study. International Journal of Nursing Studies 2016;64:86‐95. [DOI: 10.1016/j.ijnurstu.2016.09.009] - DOI - PubMed
Maly 2017 {published data only}
-
- NCT01627366. Study of survivorship care plans and outcomes in underserved breast cancer survivors. clinicaltrials.gov/ct2/show/NCT01627366 (first received 17 December 2018).
Monteil 2010 {published data only}
-
- Monteil J, Vergnenègre A, Bertin F, Dalmay F, Gaillard S, Bonnaud F, et al. Randomized follow‐up study of resected NSCLC patients: conventional versus 18F‐DG coincidence imaging. Anticancer Research 2010;30(9):3811‐6. - PubMed
-
- NCT00199615. Follow‐up of patients with curative‐intent surgical resection for NSCLC. clinicaltrials.gov/ct2/show/NCT00199615 (first received 20 December 2016).
Morrison 2018 {published data only}
-
- Morrison V, Spencer LH, Leeson S. Trial of optimal personalised care after treatment for gynaecological cancer (TOPCAT‐G): a randomised feasibility trial. Psycho‐oncology 2017;26(Suppl 2):3‐4.
-
- Morrison V, Spencer LH, Totton N, Pye K, Yeo ST, Butterworth C, et al. Trial of optimal personalised care after treatment‐gynaecological cancer (TOPCAT‐G): a randomized feasibility trial. International Journal of Gynecological Cancer 2018;28(2):401‐11. - PubMed
Murchie 2010 {published data only}
Ohlsson 1995 {published data only}
-
- Ohlsson B, Breland U, Ekberg H, Graffner H, Tranberg KG. Follow‐up after curative surgery for colorectal carcinoma. Randomized comparison with no follow‐up. Diseases of the Colon and Rectum 1995;38(6):619‐26. - PubMed
Oltra 2007 {published data only}
-
- Oltra A, Santaballa A, Montalar J, Munárriz B, Pastor M, Meseguer A, et al. Cost‐benefit evaluation of a follow‐up program in patients with advanced breast cancer. Randomized study. Revista de Oncología 1999;1(Suppl 1):165.
-
- Oltra A, Santaballa A, Munárriz B, Pastor M, Montalar J. Cost‐benefit analysis of a follow‐up program in patients with breast cancer: a randomized prospective study. Breast Journal 2007;13(6):571‐4. - PubMed
Picardi 2014 {published and unpublished data}
-
- Picardi M, Pugliese N, Cirillo M, Zeppa P, Cozzolino I, Ciancia G, et al. Advanced‐stage Hodgkin lymphoma: US/chest radiography for detection of relapse in patients in first complete remission‐‐a randomized trial of routine surveillance imaging procedures. Radiology 2014;272(1):262‐74. - PubMed
-
- Pugliese N, Cirillo M, Zeppa P, Cozzolino I, Ciancia G, Pettinato G, et al. A randomized trial of routine surveillance imaging procedures: Ultrasonography plus chest radiograph vs FDG PET/CT for detecting relapse in patients with advanced stage Hodgkin lymphoma. Haematologica 2015;100:7‐8.
-
- Pugliese N, Cirillo M, Zeppa P, Cozzolino I, Ciancia G, Pettinato G, et al. A randomized trial of routine surveillance imaging procedures: ultrasonography plus chest radiograph vs FDG PET/CT for detecting relapse in patients with advanced stage Hodgkin lymphoma. Haematologica 2014;99:398‐9. - PubMed
Pietra 1998 {published data only}
-
- Pietra N, Sarli L, Costi R, Ouchemi C, Grattarola M, Peracchia A, et al. Role of follow‐up in management of local recurrences of colorectal cancer: a prospective, randomized study. Diseases of the Colon and Rectum 1998;41(9):1127‐33. - PubMed
Primrose 2014 {published data only}
-
- Mant D, Gray A, Pugh S, Campbell H, George S, Fuller A, et al. A randomised controlled trial to assess the cost‐effectiveness of intensive versus no scheduled follow‐up in patients who have undergone resection for colorectal cancer with curative intent. Health Technology Assessment 2017;21(32):1‐86. - PMC - PubMed
-
- Mant D, Perera R, Gray A, Rose P, Fuller A, Corkhill A, et al. Effect of 3‐5 years of scheduled CEA and CT follow‐up to detect recurrence of colorectal cancer: FACS randomized controlled trial. Journal of Clinical Oncology 2013;31(15 Suppl 1):no pagination. - PubMed
-
- NCT00560365. Follow‐up study of patients who have undergone surgery for stage I, stage II, or stage III colorectal cancer. clinicaltrials.gov/ct2/show/NCT00560365 (first received 20 December 2016).
-
- Primrose JN, Fuller A, Rose P, Perera‐Salazar R, Mellor J, Corkhill A, et al. Follow‐up after colorectal cancer surgery: preliminary observational findings from the UK FACS trial. Journal of Clinical Oncology 2011;29(15 Suppl 1):3521.
Rodríguez‐Moranta 2006 {published data only}
-
- Bessa X, Saló J, Arcusa A, Elizalde JI, Boadas J, Alentorn EB, et al. Effectiveness of postoperative follow‐up in patients with colorectal cancer to detect curable recurrences. Preliminary analysis of a multicenter, randomized controlled trial [Efectividad del seguimiento postoperatorio en los pacientes con cáncer colorrectal para la detección de recidivas curables. Análisis preliminar de un estudio multicéntrico, controlado y aleatorizado]. Gastroenterologia y Hepatologia 2001;24(2):92, abstract no: 13.
-
- Bessa X, Saló J, Arcusa A, Elizalde JI, Boadas J, Alentorn EB, et al. Preliminary analysis of a multicenter controlled trial to evaluate the effectiveness of postoperative follow‐up to detect curable recurrences in patients with colorectal cancer [Análisis preliminar de un estudio multicéntrico controlado y aleatorizado destinado a evaluar la efectividad del seguimiento postoperatorio en la detección de recidivas curables en los pacientes con cáncer colorrectal]. Gastroenterologia y Hepatologia 2001;24(3):150.
-
- Rodriguez‐Moranta F, Castells A, Salo J, Arcusa A, Boadas J, Bessa V, et al. Efficacy of postoperative surveillance after radical surgery for colorectal cancer (CRC). Analysis of a prospective, multicenter, randomized controlled trial. Journal of Gastroenterology 2005;128(4 Suppl 2):Abstract W967.
-
- Rodríguez‐Moranta F, Saló J, Acrusa A, Boadas J, Bessa X, Piñol V, et al. Efficacy of postoperative surveillance in patients with colorectal cancer after radical surgery. A prospective, multicenter, randomized study. Gastroenterologia y Hepatologia 2005;28(3):197‐8, abstract no: 126.
-
- Rodríguez‐Moranta F, Saló J, Arcusa A, Boadas J, Piñol V, Bessa, X, et al. Postoperative surveillance in patients with colorectal cancer who have undergone curative resection: a prospective, multicenter, randomized, controlled trial. Journal of Clinical Oncology 2006;24(3):386‐93. - PubMed
ROGY 2015 {published and unpublished data}
-
- Rooij BH, Ezendam NP, Nicolaije KA, Lodder P, Vos MC, Pijnenborg JM, et al. Survivorship care plans have a negative impact on long‐term quality of life and anxiety through more threatening illness perceptions in gynecological cancer patients: the ROGY care trial. Quality of Life Research 2018;27(6):1533‐44. - PMC - PubMed
-
- Rooij BH, Ezendam NP, Nicolaije KA, Vos C, Pijnenborg JM, Boll D, et al. Effects of survivorship care plans on patient reported outcomes in ovarian cancer during 2‐year follow‐up ‐ The ROGY care trial. Gynecologic Oncology 2017;145(2):319‐28. - PubMed
-
- Rooij BH, Ezendam NP, Nicolaije KA, Vos C, Pijnenborg JM, Boll D, et al. Long‐term impact of survivorship care plans on ovarian cancer patient reported outcomes‐new results of the ROGY care trial. Quality of Life Research 2016;25(1 Suppl 1):64‐5.
-
- Rooij BH, Ezendam NP, Nicolaije KA, Vos MC, Pijnenborg JM, Boll D, et al. The indirect effects of survivorship care plans on quality of life, anxiety and depression‐the ROGY care trial. Supportive Care in Cancer 2017;25(2 Suppl 1):S53‐4.
-
- Rooij BH, Ezendam NP, Vos MC, Pijnenborg JM, Boll D, Kruitwagen RF, et al. Patients' information coping styles influence the benefit of a survivorship care plan in the ROGY care trial: new insights for tailored delivery. Cancer Epub 2018 Nov 30;125(5):788‐97. [DOI: 10.1002/cncr.31844] - DOI - PMC - PubMed
Rosselli Del Turco 1994 {published data only}
-
- Palli D, Russo A, Saieva C, Ciatto S, Rosselli Del Turco M, Distante V, et al. Intensive vs clinical follow‐up after treatment of primary breast cancer: 10‐year update of a randomized trial. National Research Council Project on Breast Cancer Follow‐up. JAMA 1999;281(17):1586. - PubMed
-
- Roselli Del Turco M, Palli D, Cariddi A, Ciatto S, Pacini P, Distante V. The efficacy of intensive follow‐up testing in breast cancer cases. Annals of Oncology 1995;6(Suppl 2):37‐9. - PubMed
-
- Rosselli Del Turco M, Palli D, Cariddi A, Ciatto S, Pacini P, Distante V. Intensive diagnostic follow‐up after treatment of primary breast cancer. A randomized trial. National Research Council Project on Breast Cancer follow‐up. JAMA 1994;271(20):1593‐7. - PubMed
Ruddy 2016 {published data only}
-
- Ruddy KJ, Baker EL, Guo H, Goldstein MJ, Winer EP, Shulman LN, et al. Randomized trial evaluating a coordinated survivorship care program for early stage breast cancer. Cancer Research 2013;73(24 Suppl 1):OT2‐5‐02.
-
- Ruddy KJ, Guo H, Baker E, Goldstein M, Mullaney EE, Shulman LN, et al. Coordinated follow‐up breast cancer survivorship care program study. Journal of Clinical Oncology 2015;33(15 Suppl 1):e17727.
-
- Ruddy KJ, Guo H, Baker EL, Goldstein MJ, Mullaney EE, Shulman LN, et al. Randomized phase 2 trial of a coordinated breast cancer follow‐up care program. Cancer 2016;122:3546‐54. - PubMed
Rustin 2007 {published data only}
-
- Goodman A. How often to follow up? Study shows two CT scans as good as five for low‐risk testicular cancer. Oncology Times 2007;29(12):54‐5.
-
- NCT00003420. CT scans in treating patients with stage I testicular cancer after undergoing orchiectomy. clinicaltrials.gov/ct2/show/NCT00003420 (first received 20 December 2016).
-
- Rustin GJ, Mead GM, Stenning SP, Vasey PA, Aass N, Huddart RA, et al. Randomized trial of two or five computed tomography scans in the surveillance of patients with stage I nonseminomatous germ cell tumors of the testis: Medical Research Council Trial TE08, ISRCTN56475197‐‐the National Cancer Research Institute Testis Cancer Clinical Studies Group. Journal of Clinical Oncology 2007;25(11):1310‐5. - PubMed
Schoemaker 1998 {published data only}
-
- Schoemaker D, Black R, Giles L, Toouli J. Yearly colonoscopy, liver CT, and chest radiography do not influence 5‐year survival of colorectal cancer patients. Gastroenterology 1998;114(1):7‐14. - PubMed
-
- Schoemaker D, Black R, Giles L, Toouli J, Alcorn J. Addition of intensive follow‐up procedures to standard follow‐up did not improve survival in patients with colorectal cancer. Evidence‐Based Medicine 1998;3(5):140.
Secco 2002 {published data only}
-
- Secco GB, Fardelli R, Gianquinto D, Bonfante P, Baldi E, Ravera G, et al. Efficacy and cost of risk‐adapted follow‐up in patients after colorectal cancer surgery: a prospective, randomized and controlled trial. European Journal of Surgical Oncology 2002;28(4):418‐23. - PubMed
Sheppard 2009 {published data only}
Sobhani 2008 {published data only}
Sobhani 2018 {published data only}
-
- NCT00624260. Impact of PET Scan on the curative strategy of colo‐rectal cancers : a randomized study. clinicaltrials.gov/ct2/show/NCT00624260 (first received 20 December 2016).
-
- Sobhani I, Baumgaertner I, Itti E, Luciani A, Layese R, Natella PA, et al. Colorectal cancer (CRC) patients surveyed by 18FDGPET‐CT (PET‐CT): an openlabel multicenter randomized trial (NCT 00624260). Journal of Clinical Oncology 2017;35(15 Suppl 1):no pagination.
-
- Sobhani I, Baumgaertner I, Tounigand C, Ette E, Brunetti F, Gagniere C, et al. Follow‐up of colorectal cancer (CRC) patients including 18FDGPET‐CT (PET‐CT): an open‐label multicenter randomized trial (clinical trial: NCT 00624260). United European Gastroenterology Journal 2017;5(5 Suppl 1):A13.
Van der Meulen 2013 {published data only}
-
- Meulen I, May A, Ros W, Oosterom M, Hordijk GJ, Koole R, et al. Effects of a nurse‐led psychosocial intervention on depressive symptoms and health‐related quality of life in patients with head and neck cancer: a randomized controlled trial. Psycho‐oncology 2013;22(Suppl 3):17.
-
- Meulen IC, May AM, Ros WJ, Oosterom M, Hordijk GJ, Koole R, et al. One‐year effect of a nurse‐led psychosocial intervention on depressive symptoms in patients with head and neck cancer: a randomized controlled trial. Oncologist 2013;18(3):336‐44. [DOI: 10.1634/theoncologist.2012-0299] - DOI - PMC - PubMed
Verschuur 2009 {published data only}
-
- Polinder S, Verschuur EM, Siersema PD, Kuipers, EJ, Steyerberg EW. Cost comparison study of two different follow‐up protocols after surgery for oesophageal cancer. European Journal of Cancer 2009;45(12):2110‐5. - PubMed
Wang 2009 {published data only}
-
- Wang T, Cui Y, Huang WS, Deng YH, Gong W, Li CJ, et al. The role of postoperative colonoscopic surveillance after radical surgery for colorectal cancer: a prospective, randomized clinical study. Gastrointestinal Endoscopy 2009;69(3 Pt 2):609‐15. - PubMed
Wattchow 2006 {published data only}
-
- Gall CA, Weller D, Esterman A, Pilotto L, McGorm K, Hammett Z, et al. Patient satisfaction and health‐related quality of life after treatment for colon cancer. Diseases of the Colon and Rectum 2007;50(6):801‐9. - PubMed
-
- Wattchow DA, Weller D, Pilotto L, Estermann A. Randomized controlled trial (RCT) comparing hospital and general practice based follow‐up of patients with colon cancer. ANZ Journal of Surgery 2002;72 (Suppl):A22.
-
- Wattchow DA, Weller DP, Lewis MC, Esterman A, Pilotto LS, McGorm K, et al. Randomised controlled trial of general practitioner compared to surgeon follow‐up of patients with colon cancer. Colorectal Disease 2005;7(Suppl 1):82.
Westeel 2012 {published and unpublished data}
-
- NCT00198341. Post‐surgical non‐small cell lung cancer (NSCLC) follow‐up. clinicaltrials.gov/ct2/show/NCT00198341 (first received 20 December 2016).
-
- Westeel V. Follow‐up of the patient after curative treatment. Lung Cancer 2011;71:S10‐1.
-
- Westeel V. Surveillance and second primary malignancies in lung cancer survivors. Journal of Thoracic Oncology 2018;13(10 Suppl):S277‐8.
-
- Westeel V, Barlesi F, Domas J, Girard P, Foucher P, Lafitte JJ, et al. Postoperative follow‐up of lung cancer: randomized trial comparing two follow‐up programs in completely resected non‐small cell lung cancer (IFCT‐0302). Journal of Clinical Oncology 2012;30(15 Suppl 1):no pagination.
-
- Westeel V, Barlesi F, Foucher P, Lafitte J, Domas J, Girard P, et al. Recurrences and 2nd primary cancers in the IFCT‐0302 trial assessing a CT‐scan‐based follow‐up after lung cancer surgery. Journal of Thoracic Oncology 2017;12(11 Suppl 2):S1788‐9.
Wille‐Jorgensen 2018 {published data only}
-
- NCT00225641. Assessment of frequency of surveillance after curative resection in patients with stage II and III colorectal cancer. clinicaltrials.gov/ct2/show/NCT00225641 (first received 20 December 2016).
-
- Wille‐Jorgensen P, Syk I, Smedh K, Laurberg S, Nielsen DT, Pahlman L, et al. Intensity of follow up after surgery for colorectal cancer. Colorectal Disease 2014;16:93.
-
- Wille‐Jorgensen P, Syk I, Smedh K, Laurberg S, Nielsen DT, Petersen SH, et al. Effect of more vs less frequent follow‐up testing on overall and colorectal cancer‐specific mortality in patients with stage II or III colorectal cancer: the COLOFOL randomized clinical trial. JAMA 2018;319(20):2095‐103. - PMC - PubMed
Young 2013 {published data only}
-
- ACTRN12608000252314. Cancer care after surgery – the CONNECT study. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=82819 (first received 20 December 2016).
-
- Harrison JD, Young JM, Solomon MJ, Butow PN, Secomb R, Masya L. Connect: a pilot RCT to improve outcomes for people with colorectal cancer. Asia‐Pacific Journal of Clinical Oncology 2009;5:A159.
-
- Harrison JD, Young JM, Solomon MJ, Butow PN, Secomb R, Masya L. Randomized pilot evaluation of the supportive care intervention "CONNECT" for people following surgery for colorectal cancer. Diseases of the Colon and Rectum 2011;54(5):622‐31. - PubMed
-
- Young JM, Butow PN, Walsh J, Durcinoska I, Dobbins TA, Rodwell L, et al. Multicenter randomized trial of centralized nurse‐led telephone‐based care coordination to improve outcomes after surgical resection for colorectal cancer: the CONNECT intervention. Journal of Clinical Oncology 2013;31(28):3585‐91. [DOI: 10.1200/JCO.2012.48.1036] - DOI - PubMed
References to studies excluded from this review
Baildam 2002 {published data only}
-
- Baildam A. Specialist nurse‐led clinics for breast cancer patients. Unknown 2000.
-
- Baildam A, Keeling F, Noblet M, Thomson L, Bundred N, Hopwood P. Nurse‐led follow‐up clinics for women treated for primary breast cancer: a randomised controlled trial. European Journal of Cancer 2002;38(3):136.
-
- Baildam AD, Keeling F, Thompson L, Bundred N, Hopwood P. Nurse‐ led surgical follow up clinics for women treated for breast cancer ‐ a randomised controlled trial. Unknown 2004.
Chang 2013 {published data only}
-
- Chang SN, Shun SC, Lai YH, Chen CH, Sheu JC. Effects of telephone follow‐up consultations on discharged patients with liver cancer following non‐surgical treatment. Supportive Care in Cancer 2013;21:S102.
Cruickshank 2015 {published data only}
-
- Cruickshank S, Barber DM, Kennedy C, Rowat A, Small R. Using a needs assessment tool in breast cancer follow‐up. European Journal of Cancer Supplement 2010;8(6):36.
-
- Cruickshank S, Barber M, Donaldson J, Raeside R, Gray M. A randomised controlled trial to assess the effectiveness of using patient reported needs and psychological information to guide care in a breast cancer follow‐up clinic. Cancer Nursing 2015;38(4 Suppl 1):S34‐5.
Ebell 1998 {published data only}
-
- Ebell M. Does intensive follow‐up improve outcomes for patients with colon cancer?. Evidence‐Based Practice 1999;2(1):no pagination.
-
- Ebell M. What is the role of colonoscopy, liver CT, and chest radiography in the follow‐up of patients with colorectal cancer?. Evidence‐Based Practice 1998;1(5):no pagination.
Faithfull 2001 {published data only}
Gulliford 1997 {published data only}
Haq 2015 {published data only}
-
- Haq R, Brezden‐Masley C, Lee R, Richter S, George RL, Simpson J, et al. Results of a randomized controlled study of personalized care plans in breast cancer survivors from a single institution. Journal of Clinical Oncology 2015;33(28 Suppl 1):109.
Helgesen 2000 {published data only}
-
- Helgesen F, Andersson SO, Gustafsson O, Varenhorst E, Gobén B, Carnock S, et al. Follow‐up of prostate cancer patients by on‐demand contacts with a specialist nurse: a randomized study. Scandinavian Journal of Urology and Nephrology 2000;34(1):55‐61. - PubMed
Holtedahl 2005 {published data only}
-
- Holtedahl K, Norum J, Anvik T, Richardsen E. Do cancer patients benefit from short‐term contact with a general practitioner following cancer treatment? A randomised, controlled study. Supportive Care in Cancer 2005;13(11):949‐56. - PubMed
Jakobsen 2013 {published data only}
-
- Jakobsen E, Dan Joergensen O, Ladegaard L, Petersen S, Aagaard I, Mikkelsen P, et al. Postoperative rehabilitation and follow‐up‐a randomized study. Journal of Thoracic Oncology 2013;8:S390‐1.
Jefford 2011 {published data only}
-
- Jefford M, Lotfi‐Jam K, Baravelli C, Grogan S, Rogers M, Krishnasamy M, et al. Development and pilot testing of a nurse‐led posttreatment support package for bowel cancer survivors. Asia‐Pacific Journal of Clinical Oncology 2011;34(3):E1‐10. - PubMed
Kessler 2013 {published data only}
-
- Kessler L, Loggers E, Gatsonis C, Patrick D, Sullivan S, Hanash S, et al. Surveillance trial to improve longevity in lung cancer patients (STILL). American Journal of Respiratory and Critical Care Medicine 2013;187:ASS39.
Kew 2006 {published data only}
-
- Kew FM, Cruickshank DJ, Wright L, McNeil J, Russell D, Russell IT. Follow‐up in gynaecological cancer units: randomised controlled trial. Evaluation, feasibility study. Annual Meeting of the British Gynaecological Cancer Society. 2006:84.
-
- Kew FM, Fisher A, Galaal KA, Wright L, McNeil J, Cruickshank DJ. Use of a telephone helpline for follow up after gynaecological cancer. Annual Meeting of the British Gynaecological Cancer Society. 2006:85.
Lanceley 2017 {published data only}
-
- Lanceley A, Berzuini C, Burnell M, Gessler S, Morris S, Ryan A, et al. Ovarian cancer follow‐up: a preliminary comparison of 2 approaches. International Journal of Gynecological Cancer 2017;27(1):59‐68. - PubMed
Lavau‐Denes 2013 {published data only}
-
- Lavau‐Denes S, Monteil J, Zasadny X, Duluc M, Mundler O, Smith D, et al. Positron emission tomography (PET) interest in the follow‐up of colorectal cancer stage II and III: PETCOLON study. European Journal of Cancer 2013;49:S519.
-
- NCT00199654. Positron emission tomography (PET) interest in the follow up of colorectal cancer stage II and III. clinicaltrials.gov/ct2/show/NCT00199654 (first received 20 December 2016).
Lyu 2016 {published data only}
Majhail 2019 {published data only}
-
- Majhail NS, Murphy EA, Laud P, Preussler J, Denzen E, Adams A, et al. Individualized treatment summaries and survivorship care plans (SCPs) for hematopoietic cell transplant (HCT) survivors reduces cancer treatment distress in a randomized, multicenter study. Blood 2017;130 Suppl 1:no pagination.
Mathew 2014 {published data only}
-
- Mathew J, Prinsloo P, Agrawal A, Gutteridge E, Marenah C, Robertson JF, et al. Pilot randomised study of early intervention based on tumour markers in the follow‐up of patients with primary breast cancer. Breast 2014;23(5):567‐72. - PubMed
Moore 2002 {published data only}
NCT00049465 {published data only}
-
- NCT00049465. Standard follow‐up compared with extended follow‐up in treating patients who have undergone stem cell transplantation for cancer. clinicaltrials.gov/ct2/show/NCT00049465 (first received 20 December 2016).
NCT01824745 {published data only}
-
- NCT01824745. Survivorship care planning in improving the quality of life in breast cancer survivors. clinicaltrials.gov/ct2/show/NCT01824745 (first received 20 December 2016).
NCT01973946 {published data only}
-
- NCT01973946. Cancer symptom monitoring telephone system with nurse practitioner (NP) follow up. clinicaltrials.gov/ct2/show/NCT01973946 (first received 20 December 2016).
NCT01993901 {published data only}
-
- NCT01993901. The influence of CSRT‐led telephone follow‐up on ESAS scores in patients who have been treated with lung SBRT. clinicaltrials.gov/ct2/show/NCT01993901 (first received 20 December 2016).
NCT02200133 {published data only}
-
- NCT02200133. Randomized study of individualized care plans for hematopoietic cell transplant survivors. clinicaltrials.gov/ct2/show/NCT02200133 (first received 20 December 2016).
NCT02209415 {published data only}
-
- NCT02209415. EUS‐based follow‐up on R0‐resected patients for esophageal, gastric and pancreatic cancer (EUFURO). clinicaltrials.gov/ct2/show/NCT02209415 (first received 20 December 2016).
NCT02361099 {published data only}
-
- NCT02361099. SENTINEL: impact of the use of a web‐application for the detection of lung cancer relapse. clinicaltrials.gov/ct2/show/NCT02361099 (first received 20 December 2016).
NCT02655068 {published data only}
-
- NCT02655068. Phase III trial of PET/CT vs. CT surveillance for head and neck cancer. clinicaltrials.gov/ct2/show/NCT02655068 (first received 20 December 2016).
NCT03056469 {published data only}
-
- NCT03056469. Patient‐reported outcomes integrated in the follow‐up of patients with hematological cancer. clinicaltrials.gov/ct2/show/NCT03056469 (first received 17 December 2018).
NCT03125070 {published data only}
-
- NCT03125070. Self‐management program and survivorship care plan in improving the health of cancer survivors after stem cell transplant. clinicaltrials.gov/ct2/show/NCT03125070 (first received 17 December 2018).
NCT03271099 {published data only}
-
- NCT03271099. Patient navigation in cancer survivorship at a safety net institution. clinicaltrials.gov/ct2/show/NCT03271099 (first received 17 December 2018).
NCT03360994 {published data only}
-
- NCT03360994. WATChmAN virtual testicular cancer clinic. clinicaltrials.gov/ct2/show/NCT03360994 (first received 17 December 2018).
NCT03424837 {published data only}
-
- NCT03424837. A survivorship care plan and embedded navigation tool. clinicaltrials.gov/ct2/show/NCT03424837 (first received 17 December 2018).
NCT03608410 {published data only}
-
- NCT03608410. Intensified follow‐up of lung cancer using weekly questionnaires via the internet. clinicaltrials.gov/ct2/show/NCT03608410 (first received 17 December 2018).
NCT03618017 {published data only}
-
- NCT03618017. The ConnectedCancerCare pilot study (CCC). clinicaltrials.gov/ct2/show/NCT03618017 (first received 17 December 2018).
Parker 2018 {published data only}
Ploos van Amstel 2016 {published data only}
Puri 2018 {published data only}
-
- Puri A, Gulia A. Trial for optimal surveillance in sarcoma. Pediatric Blood and Cancer 2011;57 (5):717.
-
- Puri A, Ranganathan P, Gulia A, Crasto S, Hawaldar R, Badwe RA. Does a less intensive surveillance protocol affect the survival of patients after treatment of a sarcoma of the limb? Updated results of the randomized TOSS study. Bone Joint Journal 2018;100‐B(2):262‐8. - PubMed
Rogers 2018 {published data only}
Rustin 2010 {published data only}
-
- Rustin GJ, Burg ME, Griffin CL, Guthrie D, Lamont A, Jayson GC, et al. Early versus delayed treatment of relapsed ovarian cancer (MRC OV05/EORTC 55955): a randomised trial. Lancet 2010;376(9747):1155‐63. - PubMed
Samawi 2017 {published data only}
-
- Samawi H, Yin Y, Lim HJ, Renouf DJ, Cheung WY. Primary care vs. oncology‐driven surveillance following adjuvant chemotherapy in resected pancreas cancer. Journal of Clinical Oncology 2017;35(15 Suppl 1):no pagination.
Skolarus 2017 {published data only}
Smith 2016 {published data only}
Song 2018 {published data only}
-
- Song L, Dunlap KL, Tan X, Chen RC, Nielsen ME, Rabenberg RL, et al. Enhancing survivorship care planning for patients with localized prostate cancer using a couple‐focused mHealth symptom self‐management program: protocol for a feasibility study. JMIR Research Protocols 2018;7(2):e51. - PMC - PubMed
-
- Song L, Mayer DK, Tan X, Nielsen ME, Chen RC, Northouse LL. Feasibility and preliminary effects: using a symptom self management mhealth program to enhance survivorship care plans for men with localized prostate cancer and their partners. Supportive Care in Cancer 2018;26((Suppl 3)):S394.
Stanciu 2015 {published data only}
-
- Stanciu MA, Morris C, Makin M, Watson E, Bulger J, Evans R, et al. A pilot randomised controlled trial of personalised care after treatment for prostate cancer (TOPCAT‐P): nurse‐led holistic‐needs assessment and individualised psychoeducational intervention: study protocol. BMJ Open 2015;5(6):e008470. - PMC - PubMed
Strand 2011 {published data only}
-
- Strand E, Nygren I, Bergkvist L, Smedh K. Nurse or surgeon follow‐up after rectal cancer: a randomized trial. Colorectal Disease 2011;13(9):999‐1003. - PubMed
Van Rhijn 2011 {published data only}
-
- Rhijn BW. Prospective trial to identify optimal bladder cancer surveillance protocol: reducing costs while maximizing sensitivity. BJU International 2011;108(7):1123‐4. - PubMed
-
- Aa MN, Steyerberg EW, Bangma CH, Rhijn BW, Zwarthoff EC, Kwast TH. Cystoscopy revisited as the gold standard for detection of bladder cancer recurrence: Diagnostic review bias in a randomised prospective trial. Journal of Urology 2009;181(4 Suppl 1):690. - PubMed
Verberne 2015 {published data only}
-
- Verberne C, Doornbos PM, Grossmann I, Bock GH, Wiggers T. Intensified follow‐up in colorectal cancer patients using frequent carcino‐embryonic antigen (CEA) measurements and CEA‐triggered imaging. European Journal of Cancer 2013;49:S480. - PubMed
-
- Verberne C, Heuvel E, Bock GH, Grossmann I, Wiggers T. Intensified follow‐up in colorectal cancer patients using frequent carcinoembryonic (CEA) measurements and CEA‐triggered Imaging. Annals of Surgical Oncology 2014;21(1 Suppl 1):S6.
-
- Verberne C, Wiggers T, Bock GH, Grossmann I. Sensitivity and specificity of CEA in colorectal cancer follow‐up. European Journal of Surgical Oncology 2014;40(11):S104.
-
- Verberne C, Zhan Z, Bock G, Wiggers T. Intensifying colorectal cancer follow‐up ‐ Survival analysis of the randomized multicenter CEAwatch trial. European Journal of Surgical Oncology 2016;42(9):S106.
-
- Verberne CJ, Wiggers T, Grossmann I, Bock GH, Vermeulen KM. Cost‐effectiveness of a carcinoembryonic antigen (CEA) based follow‐up programme for colorectal cancer (the CEA Watch trial). Colorectal Disease 2016;18(3):O91‐6. - PubMed
Visser 2015 {published data only}
-
- Visser A, Govaert P, Schlooz M, Dalen T, Jansen L, Laarhoven H, et al. Cost‐analyses of group medical consultation (GMCS) in the follow‐up of breast cancer. Psycho‐oncology 2014;23:137.
-
- Visser A, Laarhoven H, Schlooz M, Dalen T, Prins J. Evaluation of group medical consultations in the follow‐up of breast cancer: a randomized controlled pilot study. Psycho‐oncology 2013;22:344.
-
- Visser A, Laarhoven HW, Govaert PH, Schlooz MS, Jansen L, Dalen T, et al. Group medical consultations in the follow‐up of breast cancer: a randomized feasibility study. Journal of Cancer Survivorship 2015;9(3):450‐61. - PubMed
-
- Visser A, Prins J, Jansen L, Dalen T, Laarhoven H. Group medical consultations (GMCs) in combination with tablet‐based video GMCs as an alternative for individual breast cancer follow‐up visits: results from a randomized controlled trial. Psycho‐oncology 2015;24:45.
Watson 2014 {published data only}
-
- Watson EK, Shinkins B, Matheson L, Burns RM, Frith E, Neal D, et al. Supporting prostate cancer survivors in primary care: findings from a pilot trial of a nurse‐led psycho‐educational intervention (PROSPECTIV). European Journal of Oncology Nursing 2018;32:73‐81. - PubMed
Wheelock 2015 {published data only}
-
- Melisko ME, Mihalis E, Ernest M, Rugo HS, Park JW, Moasser MM, et al. A pilot study comparing a patient‐centered symptom‐reporting follow‐up program to standard care in patients who have completed the acute phase of treatment for early breast cancer. Journal of Clinical Oncology 2010;28(15 Suppl 1):TPS272.
-
- NCT01308775. Comparing (SIS.NET) to standard care in patients who have completed the acute phase of treatment for early breast cancer. clinicaltrials.gov/ct2/show/NCT01308775 (first received 20 December 2016).
-
- Wheelock A, Bock M, Mihalis E, Hwang J, Shepard NL, Rugo H, et al. Incorporation of web‐based symptom reporting and management in follow‐up (FU) care for early‐stage breast cancer. Supportive Care in Cancer 2012;20:S269‐70.
-
- Wheelock A, Bock M, Mihalis E, Shepard N, Moore DH, Ernest ML, et al. A randomized trial evaluating the integration of online questionnaires into follow‐up (FU) care for early‐stage breast cancer (ESBC). Journal of Clinical Oncology 2012;30(15 Suppl 1):no pagination.
References to ongoing studies
Bjerring 2016 {published data only}
-
- Bjerring OS, Fristrup CW, Mortensen MB. Comparison of standard follow‐up and intensive PET/CT and EUS based follow‐up in patients having radical surgery for pancreas and gastric cancer. A randomized controlled study. HPB : the Official Journal of the International Hepato Pancreato Biliary Association 2016;18:e153.
Duineveld 2015 {published data only}
-
- Duineveld LA, Wieldraaijer T, Asselt KM, Nugteren IC, Donkervoort SC, Ven AW, et al. Improving care after colon cancer treatment in The Netherlands, personalised care to enhance quality of life (I CARE study): study protocol for a randomised controlled trial. Trials 2015;16(1):284. [DOI: 10.1186/s13063-015-0798-7] - DOI - PMC - PubMed
Ezendam 2018 {published data only}
-
- Ezendam NP, Rooij BH, Kruitwagen RF, Creutzberg CL, Loon I, Boll D, et al. ENdometrial cancer SURvivors' follow‐up carE (ENSURE): less is more? Evaluating patient satisfaction and cost‐effectiveness of a reduced follow‐up schedule: study protocol of a randomized controlled trial. Trials 2018;19(1):227. - PMC - PubMed
-
- NCT02413606. ENdometrial Cancer SURvivors' Follow‐up carE (ENSURE): less is more?. clinicaltrials.gov/ct2/show/NCT02413606 (first received 17 December 2018).
Favales 2015 {published data only}
-
- Favales F, Pulice I, Consonni PV, Bossi P, Licitra L. Health and economic outcomes of two different follow up strategies in effectively cured advanced head and neck cancer patients‐ trial in progress. Annals of Oncology 2015;26(Suppl 6):vi.
-
- Licitra LF, Favales F, Sesenna E, Spriano G, Ansarin M, Benazzo M. Health and economic outcomes of two different follow up strategies in effectively cured advanced head and neck cancer patients. Journal of Clinical Oncology 2015;33(Suppl):TPS6625.
-
- Licitra LF, Favales F, Sesenna E, Spriano G, Ansarin M, Benazzo M, et al. Health and economic outcomes of two different follow up strategies in effectively cured advanced head and neck cancer patients. Journal of Clinical Oncology 2015;33(15 Suppl 1):No pagination.
-
- NCT02262221. Health and economic outcomes of two different follow up strategies in effectively cured advanced head and neck cancer. clinicaltrials.gov/ct2/show/NCT02262221 (first received 20 December 2016).
FURCA 2017 {published data only}
-
- Hovdenak Jakobsen I, Juul T, Bernstein I, Christensen P, Jensen FS, Johansen C, et al. Follow‐up after rectal cancer: developing and testing a novel patient‐led follow‐up program. Study protocol. Acta Oncologica (Stockholm, Sweden) 2017;56(2):307‐13. - PubMed
-
- NCT03622437. Individual follow‐up after rectal cancer ‐ focus on the needs of the patient (FURCA). clinicaltrials.gov/ct2/show/NCT03622437 (first received 17 December 2018).
Hojo 2015 {published data only}
-
- Hojo T, Masuda N, Mizutani T, Shibata T, Kinoshita T, Tamura K, et al. Intensive vs. standard post‐operative surveillance in high‐risk breast cancer patients (INSPIRE): Japan Clinical Oncology Group Study JCOG1204. Japanese Journal of Clinical Oncology 2015;45(10):983‐6. - PubMed
-
- JPRN‐UMIN000012429. Intensive vs. standard post‐operative surveillance in high risk breast cancer patients (JCOG1204, INSPIRE). upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000014255 (first received 20 December 2016).
Jefford 2017 {published data only}
-
- ACTRN12617000004369. The efficacy of a shared‐care model of follow‐up for survivors of colorectal cancer. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=371925 (first received 17 December 2018).
KRONOS 2017 {published data only}
-
- Barbieri E, Gion M, Mariani L, Stieber P, Rubino D, Fanti S, et al. Three‐monthly dynamic evaluation of CEA and CA15‐3 and 18‐FDG PET vs usual practice in the follow‐up of early breast cancer patients: a prospective, multicenter, randomized trial (KRONOS >= Patient‐Oriented New Surveillance‐Study Italy). Annals of Oncology 2017;28(Suppl 6):vi81.
-
- Barbieri E, Zamagni C, Gion M, Mariani L, Stieber P, Rubino D, et al. CEA, CA15.3 and 18‐FDG PET in the follow‐up of early breast cancer patients: a prospective, multicentric, randomized trial‐KRONOS patient‐oriented new surveillance study Italy. European Journal of Nuclear Medicine and Molecular Imaging 2017;44(2 Suppl 1):S657.
-
- NCT02261389. Follow‐up of early breast cancer by dynamic evaluation of CEA and CA 15.3 Followed by 18FDG‐PET. clinicaltrials.gov/ct2/show/NCT02261389 (first received 17 December 2018).
-
- Zamagni C, Gion M, Mariani L, Stieber P, Quercia S, Rubino D, et al. Three‐monthly dynamic evaluation of CEA and CA15‐3 (followed by 18‐FDG PET) vs usual practice in the follow‐up of early breast cancer (BC) patients (pts): a prospective randomized trial (KRONOS‐patient‐oriented new surveillance study, Italy). Cancer Research 2017;77(4 Suppl 1):no pagination.
-
- Zamagni C, Gion M, Mariani L, Stieber P, Rubino D, Fanti S, et al. CEA, CA15.3 and 18‐FDG PET in the follow‐up of early breast cancer (BC) patients (pts): a prospective, multicentric, randomized trial KRONOS patient oriented new surveillance study Italy. Journal of Clinical Oncology 2017;35(15 Suppl 1):no pagination.
Lepage 2015 {published data only}
-
- Lepage C, Phelip JM, Cany L, Faroux R, Manfredi S, Ain JF, et al. Effect of 5 years of imaging and CEA follow‐up to detect recurrence of colorectal cancer: The FFCD PRODIGE 13 randomised phase III trial. Digestive and Liver Disease 2015;47(7):529‐31. - PubMed
-
- Lepage C, Phelip JM, Cany L, Maillard E, Lievre A, Chatellier T, et al. Effect of 5 years of imaging and CEA follow‐up to detect recurrence of colorectal cancer‐PRODIGE 13 a FFCD and Unicancer phase III trial: baseline characteristics. Annals of Oncology 2016;27(Suppl 6):no pagination.
-
- NCT00995202. Follow‐up care with or without CEA assessments in patients who have undergone surgery for stage II or stage III colorectal cancer. clinicaltrials.gov/ct2/show/NCT00995202 (first received 20 December 2016).
Mathiesen 2014 {published data only}
-
- Mathiesen MM, Jensen PT, Hansen DG, Mogensen O. Follow‐up of endometrial cancer patients‐a randomized controlled trial comparing self‐referral with conventional follow‐up. International Journal of Gynecological Cancer 2014;24(8 Suppl 2):no pagination.
-
- NCT01853865. Follow‐up of endometrial cancer patients (OPAL). clinicaltrials.gov/ct2/show/NCT01853865 (first received 17 December 2018).
NCT01450020 {published data only}
-
- NCT01450020. Peer navigator education in improving survivorship care in African American breast cancer survivors. clinicaltrials.gov/ct2/show/NCT01450020 (first received 20 December 2016).
NCT02261389 {published data only}
-
- NCT02261389. Follow‐up of early breast cancer by dynamic evaluation of CEA and CA 15.3 followed by 18FDG‐PET. clinicaltrials.gov/ct2/show/NCT02261389 (first received 20 December 2016).
NCT02298855 {published data only}
-
- NCT02298855. Individualised versus conventional medical follow‐up for women after primary treatment for ovarian cancer. clinicaltrials.gov/ct2/show/NCT02298855 (first received 20 December 2016).
NCT02637349 {published data only}
-
- NCT02637349. Polaris Oncology Survivor Transition (POST) system. clinicaltrials.gov/ct2/show/NCT02637349 (first received 17 December 2018).
NCT02935920 {published data only}
-
- NCT02935920. A study on optimizing follow‐up for postmenopausal women with breast cancer treated with adjuvant endocrine therapy. clinicaltrials.gov/ct2/show/NCT02935920 (first received 17 December 2018).
NCT02949167 {published data only}
-
- NCT02949167. MyHealth: follow‐up after breast cancer treatment. clinicaltrials.gov/ct2/show/NCT02949167 (first received 20 December 2016).
NCT03017456 {published data only}
-
- NCT03017456. PC 360 Survivorship. clinicaltrials.gov/ct2/show/NCT03017456 (first received 17 December 2018).
NCT03116412 {published data only}
-
- NCT03116412. A randomized trial to assess the role of imaging during follow up after radical surgery of high risk melanoma (TRIM). clinicaltrials.gov/ct2/show/NCT03116412 (first received 17 December 2018).
NCT03244826 {published data only}
-
- NCT03244826. Shared care: patient‐centered management after hematopoietic cell transplantation. clinicaltrials.gov/ct2/show/NCT03244826 (first received 17 December 2018).
NCT03328247 {published data only}
-
- NCT03328247. Achieving Self‐directed Integrated Cancer Aftercare (ASICA) in melanoma. clinicaltrials.gov/ct2/show/NCT03328247 (first received 17 December 2018).
NCT03519048 {published data only}
-
- NCT03519048. Randomized multicentric comparative study between a conventional and an intensive follow up strategy after treatment of a head and neck squamous cell carcinoma. clinicaltrials.gov/ct2/show/NCT03519048 (first received 17 December 2018).
NCT03581188 {published data only}
-
- NCT03581188. A research study of patient‐led surveillance compared to clinician‐led surveillance in people treated for localised melanoma. clinicaltrials.gov/ct2/show/NCT03581188 (first received 17 December 2018).
NCT03740126 {published data only}
-
- NCT03740126. Surveillance with PET/CT and liquid biopsies of stage I‐III lung cancer patients after completion of definitive therapy (SUPE_R). clinicaltrials.gov/ct2/show/NCT03740126 (first received 17 December 2018).
Taylor 2016 {published data only}
-
- Monterosso L, Taylor KM, Bulsara C, Bulsara M, Platt V, Stratton K, et al. Development of a pilot randomised controlled trial: lymphoma nurse‐led model of survivorship care. Cancer Nursing 2016;39(6 Suppl 1):S54‐5.
-
- Taylor K, Monterosso L, Bulsara C. Qualitative results from a phase II pilot randomised controlled trial of a lymphoma nurse‐led model of survivorship care. European Journal of Oncology Nursing 2018;35:9‐14. - PubMed
-
- Taylor K, Monterosso L, Bulsara C, Bulsara M, Platt V, Stratton K, et al. Development of a phase II pilot randomised controlled trial of a lymphoma nurse‐led model of survivorship care: Care after lymphoma (CALY) trial. Supportive Care in Cancer 2016;24(1 Suppl 1):S234‐5.
-
- Taylor KM, Monterosso L, Bulsara C. Qualitative results of a phase II pilot randomised controlled trial of a lymphoma nurse‐led survivorship model of care. Asia Pacific Journal of Clinical Oncology 2018;14(Suppl 7):141. - PubMed
Turner 2014 {published data only}
Wang 2016 {published data only}
-
- Wang YA, Chen SP, Horng CF, Hsieh LL, Hsu KH, Chu CS, et al. A randomized controlled study for the long term follow‐up of breast cancer survivors: a primary care physician (PCP) coordinated care delivery model. Journal of Clinical Oncology 2016;34(3 Suppl 1):36.
Zola 2016 {published data only}
-
- NCT00916708. Trial between two follow up regimens with different test intensity in endometrial cancer treated patients. clinicaltrials.gov/ct2/show/NCT00916708 (first received 17 December 2018).
-
- Zola P, Piovano E, Ruvo D, Cuonzo D, Evangelista A, Ferrero A, et al. A multi‐center randomized controlled trial between two follow‐up regimens at different intensity in endometrial cancer patients: Totem study. International Journal of Gynecological Cancer 2016; Vol. 26:1033.
Additional references
Aaronson 1993
-
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute 1993;85(5):365‐76. [PUBMED: 8433390] - PubMed
Aslam 2016
-
- Aslam RW, Pye KL, Rai TK, Hall B, Timmis LJ, Yeo S, et al. Follow‐up strategies for women with endometrial cancer after primary treatment. Cochrane Database of Systematic Reviews 2016, Issue 10. [DOI: 10.1002/14651858.CD012386] - DOI
Aubin 2012
Barbieri 2018
-
- Barbieri M, Richardson G, Paisley S. The cost‐effectiveness of follow‐up strategies after cancer treatment: a systematic literature review. British Medical Bulletin 2018;126(1):85‐100. [PUBMED: 29659715] - PubMed
Bleyer 1990
Brennan 2011
Clarke 2014
Collins 2004
-
- Collins RF, Bekker HL, Dodwell DJ. Follow‐up care of patients treated for breast cancer: a structured review. Cancer Treatment Reviews 2004;30(1):19‐35. [PUBMED: 14766124] - PubMed
Corsaletti 2014
-
- Corsaletti BF, Proenca MG, Bisca GK, Leite JC, Bellinetti LM, Pitta F. Minimal important difference for anxiety and depression surveys after intervention to increase daily physical activity in smokers. Fisioterapia e Pesquisa 2014;21(4):359‐64.
Covidence [Computer program]
-
- Veritas Health Innovation. Covidence. Version accessed 19 December 2016. Melbourne, Australia: Veritas Health Innovation.
Davies 2011
-
- Davies NJ, Batehup L. Towards a personalised approach to aftercare: a review of cancer follow‐up in the UK. Journal of Cancer Survivorship 2011;5(2):142‐51. - PubMed
De Felice 2015
-
- Felice F, Musio D, Tombolini V. Follow‐up in head and neck cancer: a management dilemma. Advances in Otolaryngology 2015;2015:1‐4. [DOI: 10.1155/2015/703450] - DOI
Deeks 2017
-
- Deeks JJ, Higgins JP, Altman DG (editors), on behalf of the Cochrane Statistical Methods Group. Deeks JJ, Higgins JPT, Altman DG Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Dickinson 2014
EPOC 2013
-
- Cochrane Effective Practice, Organisation of Care (EPOC). Data collection form. EPOC resources for review authors. Available from epoc.cochrane.org/epoc‐specific‐resources‐review‐authors (accessed 21 October 2016).
EPOC 2015
-
- Cochrane Effective Practice, Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. EPOC Resources for review authors. Available from epoc.cochrane.org/epoc‐specific‐resources‐review‐authors (accessed 21 October 2016).
EPOC 2017
-
- Cochrane Effective Practice, Organisation of Care (EPOC). EPOC worksheets for preparing a Summary of Findings (SoF) table using GRADE. EPOC Resources for review authors. Available at: epoc.cochrane.org/resources/epoc‐resources‐review‐authors (accessed 21 October 2016).
EPOC 2018
-
- Cochrane Effective Practice, Organisation of Care (EPOC). Reporting the effects of an intervention in EPOC reviews. EPOC Resources for review authors. Available at: epoc.cochrane.org/resources/epoc‐resourcesreview‐authors (accessed 21 October 2016).
EuroQoL 1990
-
- EuroQoL Group. EuroQol ‐ a new facility for the measurement of health‐related quality of life. Health Policy 1990;16(3):199‐208. - PubMed
Goldberg 1978
-
- Goldberg DP. Manual of the General Health Questionnaire. Windsor, UK: NFER Nelson, 1978.
GRADEpro GDT 2015 [Computer program]
-
- GRADE Working Group, McMaster University (developed by Evidence Prime, Inc.). GRADEpro GDT. Version accessed 21 October 2016. Hamilton (ON): GRADE Working Group, McMaster University (developed by Evidence Prime, Inc.).
Guyatt 2008
Hall 2011
Higgins 2003
Higgins 2011a
-
- Higgins JP, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Howell 2012
-
- Howell D, Hack TF, Oliver TK, Chulak T, Mayo S, Aubin M, et al. Models of care for post‐treatment follow‐up of adult cancer survivors: a systematic review and quality appraisal of the evidence. Journal of Cancer Survivorship 2012;6(4):359‐71. [PUBMED: 22777364] - PubMed
Jeffery 2016
Jorgensen 2015
Lafranconi 2017
-
- Lafranconi A, Pylkkanen L, Deandrea S, Bramesfeld A, Lerda D, Neamtiu L, et al. Intensive follow‐up for women with breast cancer: review of clinical, economic and patient's preference domains through evidence to decision framework. Health and Quality of Life Outcomes 2017;15(1):206. [PUBMED: 29052503] - PMC - PubMed
Lanceley 2013
Landier 2009
-
- Landier W. Survivorship care: essential components and models of delivery. Oncology 2009;23(4 Suppl Nurse Ed):46‐53. [PUBMED: 19856598] - PubMed
Lewis 2009a
Lewis 2009b
-
- Lewis R, Neal RD, Williams NH, France B, Wilkinson C, Hendry M, et al. Nurse‐led vs. conventional physician‐led follow‐up for patients with cancer: systematic review. Journal of Advanced Nursing 2009;65(4):706‐23. [PUBMED: 19278415] - PubMed
Liberati 2009
Moher 2009
Moschetti 2016
Oeffinger 2006
-
- Oeffinger KC, McCabe MS. Models for delivering survivorship care. Journal of Clinical Oncology 2006;24(32):5117‐24. [PUBMED: 17093273] - PubMed
Osoba 1998
-
- Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health‐related quality‐of‐life scores. Journal of Clinical Oncology 1998;16(1):139‐44. [PUBMED: 9440735] - PubMed
Page 2017
-
- Page MJ, Higgins J, Sambunjak D, Cumpston M, Watts C. Module 5: Introduction to study quality and risk of bias. In: Cochrane Interactive Learning: Conducting an intervention review, Cochrane, 2017. Available from training.cochrane.org/interactivelearning.
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [PUBMED: 9921604] - PubMed
Patrick 2011
-
- Patrick DL, Guyatt GH, Acquadro C. Chapter 17: Patient‐reported outcomes. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Puhan 2008
R 2017 [Computer program]
-
- R Core Team. R: a language and environment for statistical computing. Version accessed 1 February 2018. Vienna, Austria: R Foundation for Statistical Computing.
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rose 2009
Sambunjak 2017
-
- Sambunjak D, Cumpston M, Watts C. Module 4: Selecting studies and collecting data. In: Cochrane Interactive Learning: Conducting an intervention review, Cochrane, 2017. Available from training.cochrane.org/interactivelearning.
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Schünemann 2017
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. on behalf of the Cochrane Applicability and Recommendations Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Schünemann 2019
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, Guyatt GH. Chapter 14: Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Draft version (29 January 2019) for inclusion in: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). London: Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Simard 2009
-
- Simard S, Savard J. Fear of Cancer Recurrence Inventory: development and initial validation of a multidimensional measure of fear of cancer recurrence. Supportive Care in Cancer 2009;17:241‐51. - PubMed
Snaith 2003
Spencer‐Thomas 2016
-
- Spencer‐Thomas O. Press release: getting the facts straight. www.owenspencer‐thomas.com (accessed 21 October 2016).
Sperduti 2013
Spielberger 1983
-
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State‐Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press, 1983.
Sterne 2011
Thompson 2002
-
- Thompson SG, Higgins JP. How should meta‐regression analyses be undertaken and interpreted?. Statistics in Medicine 2002;21(11):1559‐73. [PUBMED: 12111920] - PubMed
Tierney 2007
Wan 2014
Ware 1994
-
- Ware JE, Kosinski MA, Keller SD. SF‐36 physical and mental health summary scales: a user’s manual. Boston, Mass: The Health Institute, New England Medical Center, 1994.
Ware 1995
-
- Ware J, Kosinski M, Keller S. SF‐12: how to score the SF‐12 physical and mental health summary scales. 2nd Edition. Boston Massachusetts: The Health Institute, New England Medical Centre, 1995.
Whiting 2016
World Cancer Report 2014
-
- Stewart BW, Wild CP, (editors). World Cancer Report 2014. International Agency for Research on Cancer, WHO Press, 2014.
References to other published versions of this review
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

