Multidisciplinary team management of carcinoid heart disease
- PMID: 31751305
- PMCID: PMC6933832
- DOI: 10.1530/EC-19-0413
Multidisciplinary team management of carcinoid heart disease
Abstract
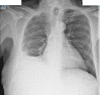
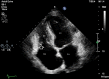
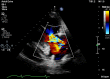
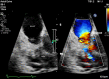
Carcinoid heart disease (CHD) is a consequence of valvular fibrosis triggered by vasoactive substances released from neuroendocrine tumours, classically in those with metastatic disease and resulting in tricuspid and pulmonary valve failure. CHD affects one in five patients who have carcinoid syndrome (CS). Valve leaflets become thickened, retracted and immobile, resulting most often in regurgitation that causes right ventricular dilatation and ultimately, right heart failure. The development of CHD heralds a significantly worse prognosis than those patients with CS who do not develop valvular disease. Diagnosis requires a low threshold of suspicion in all patients with CS, since symptoms occur late in the disease process and clinical signs are difficult to elicit. As a result, routine screening is recommended using the biomarker, N-terminal pro-natriuretic peptide, and regular echocardiography is then required for diagnosis and follow-up. There is no direct medical therapy for CHD, but the focus of non-surgical care is to control CS symptoms, reduce tumour load and decrease hormone levels. Valve surgery improves long-term outcome for those with severe disease compared to medical management, although peri-operative mortality remains at between 10 and 20% in experienced centres. Therefore, care needs to be multidisciplinary at all stages, with clear discussion with the patient and between teams to ensure optimum outcome for these often-complex patients.
Keywords: carcinoid heart disease; neuroendocrine tumours; somatostatin analogues; surgery; valvular heart disease.
Figures


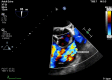

References
-
- Davar J, Connolly HM, Caplin ME, Pavel M, Zacks J, Bhattacharyya S, Cuthbertson DJ, Dobson R, Grozinsky-Glasberg S, Steeds RP, et al. Diagnosing and managing carcinoid heart disease in patients with neuroendocrine tumors: an expert statement. Journal of the American College of Cardiology 2017. 1288–1304. (10.1016/j.jacc.2016.12.030) - DOI - PubMed
-
- Rutkovskiy A, Malashicheva A, Sullivan G, Bogdanova M, Kostareva A, Stensløkken KO, Fiane A, Vaage J. Valve interstitial cells: the key to understanding the pathophysiology of heart valve calcification. Journal of the American Heart Association 2017. e006339 (10.1161/JAHA.117.006339) - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

