Mitochondrial arginase-2 is a cell‑autonomous regulator of CD8+ T cell function and antitumor efficacy
- PMID: 31751318
- PMCID: PMC6975264
- DOI: 10.1172/jci.insight.132975
Mitochondrial arginase-2 is a cell‑autonomous regulator of CD8+ T cell function and antitumor efficacy
Abstract
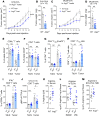
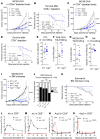
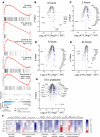
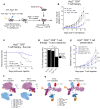
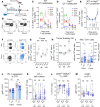
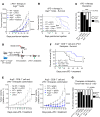
As sufficient extracellular arginine is crucial for T cell function, depletion of extracellular arginine by elevated arginase 1 (Arg1) activity has emerged as a hallmark immunosuppressive mechanism. However, the potential cell-autonomous roles of arginases in T cells have remained unexplored. Here, we show that the arginase isoform expressed by T cells, the mitochondrial Arg2, is a cell-intrinsic regulator of CD8+ T cell activity. Both germline Arg2 deletion and adoptive transfer of Arg2-/- CD8+ T cells significantly reduced tumor growth in preclinical cancer models by enhancing CD8+ T cell activation, effector function, and persistence. Transcriptomic, proteomic, and high-dimensional flow cytometry characterization revealed a CD8+ T cell-intrinsic role of Arg2 in modulating T cell activation, antitumor cytoxicity, and memory formation, independently of extracellular arginine availability. Furthermore, specific deletion of Arg2 in CD8+ T cells strongly synergized with PD-1 blockade for the control of tumor growth and animal survival. These observations, coupled with the finding that pharmacologic arginase inhibition accelerates activation of ex vivo human T cells, unveil Arg2 as a potentially new therapeutic target for T cell-based cancer immunotherapies.
Keywords: Amino acid metabolism; Cancer immunotherapy; Immunology; Mitochondria; Oncology.
Conflict of interest statement
Figures


References
-
- Barbul A, Sisto DA, Wasserkrug HL, Efron G. Arginine stimulates lymphocyte immune response in healthy human beings. Surgery. 1981;90(2):244–251. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

