Noncompletion and nonpublication of trials studying rare diseases: A cross-sectional analysis
- PMID: 31751330
- PMCID: PMC6871779
- DOI: 10.1371/journal.pmed.1002966
Noncompletion and nonpublication of trials studying rare diseases: A cross-sectional analysis
Abstract
Background: Rare diseases affect as many as 60 million people in the United States and Europe. However, most rare diseases lack effective therapies and are in critical need of clinical research. Our objective was to determine the frequency of noncompletion and nonpublication of trials studying rare diseases.
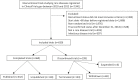
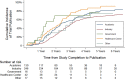
Methods and findings: We conducted a cross-sectional analysis of randomized clinical trials studying rare diseases as defined by the Genetic and Rare Disease Information Center database that were registered in ClinicalTrials.gov between January 1, 2010, and December 31, 2012, and completed or discontinued by December 31, 2014. Our main outcome measures were the frequency of trial noncompletion and, among completed studies, frequency of trial nonpublication at 2 and 4 years following trial completion. Reasons for discontinuation were extracted from the registry, and trial sponsors were contacted for additional information, as needed. Two independent investigators performed publication searches for each trial in PubMed, EMBASE, and GoogleScholar, allowing for a minimum of 45 months between trial completion and publication. When a publication could not be identified, trial sponsors were contacted to confirm publication status. The impact of funding source on trial noncompletion was assessed with multivariable logistic regression, and the effect on time to publication was examined with Cox proportional hazards regression. Control variables included intervention type, trial phase, masking, enrollment, and study population. We analyzed 659 rare disease trials accounting for 70,305 enrolled patients. Industry was the primary funder for 327 trials (49.6%) and academic institutions for 184 trials (27.9%). There were 79 trials (12.0%) focused on pediatric populations. A total of 199 trials (30.2%) were discontinued. Lack of patient accrual (n = 64, 32.1%) and informative termination (n = 41, 20.6%) were the most common reasons for trial noncompletion. Among completed trials, 306 (66.5%) remained unpublished at 2 years and 142 (31.5%) at 4 years. In multivariable analyses, industry-funded trials were less likely to be discontinued than trials funded by healthcare centers (odds ratio [OR] 2.42; 95% confidence interval [CI] 1.34-4.39, P = 0.003). We found no significant association between funding source and time to publication. A total of 18,148 patients were enrolled in trials that were discontinued or unpublished 4 years after completion. A potential limitation of our study is that certain interventional trials for rare diseases may not have been registered in ClinicalTrials.gov, in particular Phase 0 and Phase I trials, which are not required to be registered.
Conclusions: In this study, over half of clinical trials initiated for rare diseases were either discontinued or not published 4 years after completion, resulting in large numbers of patients with rare diseases exposed to interventions that did not lead to informative findings. Concerted efforts are needed to ensure that participation of patients in rare disease trials advances scientific knowledge and treatments for rare diseases.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Comment in
-
Room for improvement in clinical trials for rare diseases.Nat Rev Rheumatol. 2020 Mar;16(3):131-132. doi: 10.1038/s41584-020-0376-6. Nat Rev Rheumatol. 2020. PMID: 31996788 No abstract available.
References
-
- US Department of Health and Human Services, Food and Drug Administration. Report: Complex issues in developing drugs and biological products for rare diseases and accelerating the development of therapies for pediatric rare diseases including strategic plan: accelerating the development of therapies for pediatric rare diseases. US FDA; 2014 [cited 2019 Jan 15]. Available from: https://www.fda.gov/media/89051/download.
-
- Global Genes. RARE Diseases: Facts and Statistics Aliso Viejo, CA: Global Genes; 2015. [cited 2019 Jan 14]. https://globalgenes.org/rare-diseases-facts-statistics/
-
- Dechartres A, Riveros C, Harroch M, Faber T, Ravaud P. Characteristics and Public Availability of Results of Clinical Trials on Rare Diseases Registered at Clinicaltrials.gov. JAMA Intern Med. 2016;176: 556–558. 10.1001/jamainternmed.2016.0137 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical