Mutation That Promotes Activation of Trypsinogen Increases Severity of Secretagogue-Induced Pancreatitis in Mice
- PMID: 31751559
- PMCID: PMC7062587
- DOI: 10.1053/j.gastro.2019.11.020
Mutation That Promotes Activation of Trypsinogen Increases Severity of Secretagogue-Induced Pancreatitis in Mice
Abstract
Background & aims: Mutations in the human serine protease 1 gene (PRSS1), which encodes cationic trypsinogen, can accelerate its autoactivation and cause hereditary or sporadic chronic pancreatitis. Disruption of the locus that encodes cationic trypsinogen in mice (T7) causes loss of expression of the protein, but only partially decreases the severity of secretagogue-induced acute pancreatitis and has no effect on chronic pancreatitis. We investigated whether trypsinogen becomes pathogenic only when its activation is promoted by mutation.
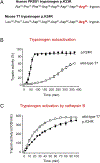
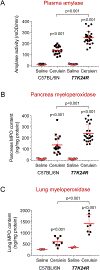
Methods: We generated mice with knock-in of the p.K24R mutation (called T7K24R mice), which is analogous to human PRSS1 mutation p.K23R. We gave T7K24R and C57BL/6N (control) mice repeated injections of cerulein to induce pancreatitis. Plasma amylase activity, pancreatic edema, and myeloperoxidase content in pancreas and lungs were quantified. We expressed mutant and full-length forms of PRSS1 in Escherichia coli and compared their autoactivation.
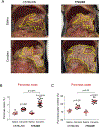
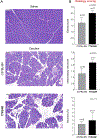
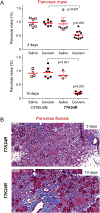
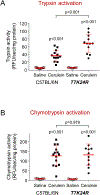
Results: The p.K24R mutation increased autoactivation of T7 5-fold. T7K24R mice developed no spontaneous pancreatitis. T7K24R mice given cerulein injections had increased pancreatic activation of trypsinogen and more edema, infiltration of lung and pancreas by inflammatory cells, and plasma amylase activity compared with control mice given cerulein injections. Injection of cerulein for 2 days induced progressive pancreatitis in T7K24R mice, but not in control mice, with typical features of chronic pancreatitis.
Conclusions: Introduction of a mutation into mice that is analogous to the p.K23R mutation in PRSS1 increases pancreatic activation of trypsinogen during secretagogue-induced pancreatitis. Higher pancreatic activity of trypsin increases the severity of pancreatitis, even though loss of trypsin activity does not prevent pancreatitis in mice.
Keywords: Genetics; Inflammation; Intrapancreatic; Mouse Model.
Copyright © 2020 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

Comment in
-
The Complex Role of Trypsin in Pancreatitis.Gastroenterology. 2020 Mar;158(4):822-826. doi: 10.1053/j.gastro.2019.12.025. Epub 2020 Jan 3. Gastroenterology. 2020. PMID: 31911102 No abstract available.
References
-
- Whitcomb DC, Gorry MC, Preston RA, Furey W, Sossenheimer MJ, Ulrich CD, Martin SP, Gates LK Jr, Amann ST, Toskes PP, Liddle R, McGrath K, Uomo G, Post JC, Ehrlich GD. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet 1996, 14:141–145 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

