Cognitive rigidity and BDNF-mediated frontostriatal glutamate neuroadaptations during spontaneous nicotine withdrawal
- PMID: 31752015
- PMCID: PMC7075915
- DOI: 10.1038/s41386-019-0574-6
Cognitive rigidity and BDNF-mediated frontostriatal glutamate neuroadaptations during spontaneous nicotine withdrawal
Abstract
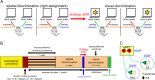
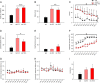
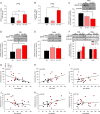
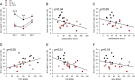
Cognitive flexibility is the ability to switch strategic responses adaptively in changing environments. Cognitive rigidity imposed by neural circuit adaptations during nicotine abstinence may foster maladaptive nicotine taking in addicts. We systematically examined the effects of spontaneous withdrawal in mice exposed to either nicotine (6.3 or 18 mg/kg/day) or saline for 14 days on cognitive flexibility using an operant strategy set-shifting task. Because frontostriatal circuits are critical for cognitive flexibility and brain-derived neurotrophic factor (BDNF) modulates glutamate plasticity in these circuits, we also explored the effects of nicotine withdrawal on these neurochemical substrates. Mice undergoing nicotine withdrawal required more trials to attain strategy-switching criterion. Error analysis show that animals withdrawn from both nicotine doses committed higher perseverative errors, which correlated with measures of anxiety. However, animals treated with the higher nicotine dose also displayed more strategy maintenance errors that remained independent of negative affect. BDNF mRNA expression increased in the medial prefrontal cortex (mPFC) following nicotine withdrawal. Surprisingly, BDNF protein declined in mPFC but was elevated in dorsal striatum (DS). DS BDNF protein positively correlated with perseverative and maintenance errors, suggesting mPFC-DS overflow of BDNF during withdrawal. BDNF-evoked glutamate release and synapsin phosphorylation was attenuated within DS synapses, but enhanced in the nucleus accumbens, suggesting a dichotomous role of BDNF signaling in striatal regions. Taken together, these data suggest that spontaneous nicotine withdrawal impairs distinct components of cognitive set-shifting and these deficits may be linked to BDNF-mediated alterations in glutamate signaling dynamics in discrete frontostriatal circuits.
Figures
Similar articles
-
Cognitive control deficits during mecamylamine-precipitated withdrawal in mice: Possible links to frontostriatal BDNF imbalance.Neurobiol Learn Mem. 2016 Feb;128:110-6. doi: 10.1016/j.nlm.2016.01.003. Epub 2016 Jan 13. Neurobiol Learn Mem. 2016. PMID: 26775017 Free PMC article.
-
Effects of chronic low- and high-dose nicotine on cognitive flexibility in C57BL/6J mice.Behav Brain Res. 2013 Feb 1;238:134-45. doi: 10.1016/j.bbr.2012.10.032. Epub 2012 Oct 24. Behav Brain Res. 2013. PMID: 23103711 Free PMC article.
-
Impact of partial dopamine depletion on cognitive flexibility in BDNF heterozygous mice.Psychopharmacology (Berl). 2016 Apr;233(8):1361-75. doi: 10.1007/s00213-016-4229-6. Epub 2016 Feb 10. Psychopharmacology (Berl). 2016. PMID: 26861892 Free PMC article.
-
Cocaine self-administration causes signaling deficits in corticostriatal circuitry that are reversed by BDNF in early withdrawal.Brain Res. 2015 Dec 2;1628(Pt A):82-7. doi: 10.1016/j.brainres.2014.09.050. Epub 2014 Sep 28. Brain Res. 2015. PMID: 25268928 Free PMC article. Review.
-
Interactions of Glutamatergic Neurotransmission and Brain-Derived Neurotrophic Factor in the Regulation of Behaviors after Nicotine Administration.Int J Mol Sci. 2019 Jun 16;20(12):2943. doi: 10.3390/ijms20122943. Int J Mol Sci. 2019. PMID: 31208140 Free PMC article. Review.
Cited by
-
Distinct patterns of prefrontal cortical disengagement during inhibitory control in addiction: A meta-analysis based on population characteristics.Neurosci Biobehav Rev. 2021 Aug;127:255-269. doi: 10.1016/j.neubiorev.2021.04.028. Epub 2021 Apr 29. Neurosci Biobehav Rev. 2021. PMID: 33933507 Free PMC article. Review.
-
Multidimensional Intersection of Nicotine, Gene Expression, and Behavior.Front Behav Neurosci. 2021 Mar 22;15:649129. doi: 10.3389/fnbeh.2021.649129. eCollection 2021. Front Behav Neurosci. 2021. PMID: 33828466 Free PMC article.
-
Nitric Oxide Linked to mGluR5 Upregulates BDNF Synthesis by Activating MMP2 in the Caudate and Putamen after Challenge Exposure to Nicotine in Rats.Int J Mol Sci. 2022 Sep 19;23(18):10950. doi: 10.3390/ijms231810950. Int J Mol Sci. 2022. PMID: 36142895 Free PMC article.
-
Glial cells as therapeutic targets for smoking cessation.Neuropharmacology. 2020 Sep 15;175:108157. doi: 10.1016/j.neuropharm.2020.108157. Epub 2020 May 24. Neuropharmacology. 2020. PMID: 32461156 Free PMC article. Review.
-
Convergent actions of stress and stimulants via epigenetic regulation of neural circuitry.Trends Neurosci. 2022 Dec;45(12):955-967. doi: 10.1016/j.tins.2022.10.001. Epub 2022 Oct 22. Trends Neurosci. 2022. PMID: 36280459 Free PMC article. Review.
References
-
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

