Mitochondrial Mass Assessment in a Selected Cell Line under Different Metabolic Conditions
- PMID: 31752092
- PMCID: PMC6912592
- DOI: 10.3390/cells8111454
Mitochondrial Mass Assessment in a Selected Cell Line under Different Metabolic Conditions
Abstract
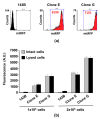
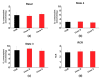
Changes of quantity and/or morphology of cell mitochondria are often associated with metabolic modulation, pathology, and apoptosis. Exogenous fluorescent probes used to investigate changes in mitochondrial content and dynamics are strongly dependent, for their internalization, on the mitochondrial membrane potential and composition, thus limiting the reliability of measurements. To overcome this limitation, genetically encoded recombinant fluorescent proteins, targeted to different cellular districts, were used as reporters. Here, we explored the potential use of mitochondrially targeted red fluorescent probe (mtRFP) to quantify, by flow cytometry, mitochondrial mass changes in cells exposed to different experimental conditions. We first demonstrated that the mtRFP fluorescence intensity is stable during cell culture and it is related with the citrate synthase activity, an established marker of the mitochondrial mass. Incidentally, the expression of mtRFP inside mitochondria did not alter the oxygen consumption rate under both state 3 and 4 respiration conditions. In addition, using this method, we showed for the first time that different inducers of mitochondrial mass change, such as hypoxia exposure or resveratrol treatment of cells, could be consistently detected. We suggest that transfection and selection of stable clones expressing mtRFP is a reliable method to monitor mitochondrial mass changes, particularly when pathophysiological or experimental conditions change ΔΨm, as it occurs during mitochondrial uncoupling or hypoxia/anoxia conditions.
Keywords: cancer cells; flow cytometry; hypoxia; mitochondrial mass; mtRFP; resveratrol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Malena A., Pantic B., Borgia D., Sgarbi G., Solaini G., Holt I.J., Spinazzola A., Perissinotto E., Sandri M., Baracca A., et al. Mitochondrial quality control: Cell-type-dependent responses to pathological mutant mitochondrial DNA. Autophagy. 2016;12:2098–2112. doi: 10.1080/15548627.2016.1226734. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

