Current and Future Concepts for the Treatment of Impaired Fracture Healing
- PMID: 31752267
- PMCID: PMC6888215
- DOI: 10.3390/ijms20225805
Current and Future Concepts for the Treatment of Impaired Fracture Healing
Abstract
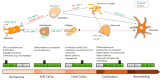
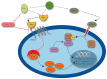
Bone regeneration represents a complex process, of which basic biologic principles have been evolutionarily conserved over a broad range of different species. Bone represents one of few tissues that can heal without forming a fibrous scar and, as such, resembles a unique form of tissue regeneration. Despite a tremendous improvement in surgical techniques in the past decades, impaired bone regeneration including non-unions still affect a significant number of patients with fractures. As impaired bone regeneration is associated with high socio-economic implications, it is an essential clinical need to gain a full understanding of the pathophysiology and identify novel treatment approaches. This review focuses on the clinical implications of impaired bone regeneration, including currently available treatment options. Moreover, recent advances in the understanding of fracture healing are discussed, which have resulted in the identification and development of novel therapeutic approaches for affected patients.
Keywords: biomaterials; fracture healing; non-union; osteoanabolic molecules.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

