Multilocus Sequence Typing (MLST) and Random Polymorphic DNA (RAPD) Comparisons of Geographic Isolates of Neoparamoeba perurans, the Causative Agent of Amoebic Gill Disease
- PMID: 31752364
- PMCID: PMC6963586
- DOI: 10.3390/pathogens8040244
Multilocus Sequence Typing (MLST) and Random Polymorphic DNA (RAPD) Comparisons of Geographic Isolates of Neoparamoeba perurans, the Causative Agent of Amoebic Gill Disease
Abstract
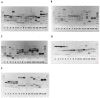
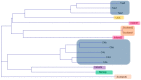
Neoparamoba perurans, is the aetiological agent of amoebic gill disease (AGD), a disease that affects farmed Atlantic salmon worldwide. Multilocus sequence typing (MLST) and Random Amplified Polymorphic DNA (RAPD) are PCR-based typing methods that allow for the highly reproducible genetic analysis of population structure within microbial species. To the best of our knowledge, this study represents the first use of these typing methods applied to N. perurans with the objective of distinguishing geographical isolates. These analyses were applied to a total of 16 isolates from Australia, Canada, Ireland, Scotland, Norway, and the USA. All the samples from Australia came from farm sites on the island state of Tasmania. Genetic polymorphism among isolates was more evident from the RAPD analysis compared to the MLST that used conserved housekeeping genes. Both techniques consistently identified that isolates of N. perurans from Tasmania, Australia were more similar to each other than to the isolates from other countries. While genetic differences were identified between geographical isolates, a BURST analysis provided no evidence of a founder genotype. This suggests that emerging outbreaks of AGD are not due to rapid translocation of this important salmonid pathogen from the same area.
Keywords: Amoebic Gill Disease; Paramoeba perurans; epidemiology; pathogen genetics.
Conflict of interest statement
Funding: This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 634429 (ParaFishControl). J.C.J-M research travel was supported by Australian Society for Parasitology travel grants, including JD Smyth Postgraduate Travel Award in 2016, Fisheries Society for Fish Biology Travel Grant and UTAS Postgraduate Travel Grant.
Figures



References
-
- Munday B.L. Diseases of salmonids. In: Humphrey J.D., Langdon J.S., editors. Proceedings of the Workshop on Disease of Australian Fish and Shellfish. Department of Agriculture and Rural Affairs; Benalla, Australia: 1986. pp. 127–141.
-
- Kent M.L., Sawyer T.K., Hedrick R.P. Paramoeba pemaquidensis (Sarcomastigophora:Paramoebidae) infestation of the gills of coho salmon Oncorhynchus kisutch reared in sea water. Diseases Aquati. Org. 1988;5:163–169. doi: 10.3354/dao005163. - DOI
-
- Oldham T., Rodger H., Nowak B.F. Incidence and distribution of amoebic gill disease (AGD)—An epidemiological review. Aquaculture. 2016;457:35–42. doi: 10.1016/j.aquaculture.2016.02.013. - DOI
LinkOut - more resources
Full Text Sources

