Redox Regulation and Oxidative Stress: The Particular Case of the Stallion Spermatozoa
- PMID: 31752408
- PMCID: PMC6912273
- DOI: 10.3390/antiox8110567
Redox Regulation and Oxidative Stress: The Particular Case of the Stallion Spermatozoa
Abstract
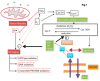
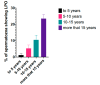
Redox regulation and oxidative stress have become areas of major interest in spermatology. Alteration of redox homeostasis is recognized as a significant cause of male factor infertility and is behind the damage that spermatozoa experience after freezing and thawing or conservation in a liquid state. While for a long time, oxidative stress was just considered an overproduction of reactive oxygen species, nowadays it is considered as a consequence of redox deregulation. Many essential aspects of spermatozoa functionality are redox regulated, with reversible oxidation of thiols in cysteine residues of key proteins acting as an "on-off" switch controlling sperm function. However, if deregulation occurs, these residues may experience irreversible oxidation and oxidative stress, leading to malfunction and ultimately death of the spermatozoa. Stallion spermatozoa are "professional producers" of reactive oxygen species due to their intense mitochondrial activity, and thus sophisticated systems to control redox homeostasis are also characteristic of the spermatozoa in the horse. As a result, and combined with the fact that embryos can easily be collected in this species, horses are a good model for the study of redox biology in the spermatozoa and its impact on the embryo.
Keywords: equine; horses; oxidative stress; reactive oxygen species (ROS); redox regulation; spermatozoa.
Conflict of interest statement
The authors declare that there is no conflict of interest that may affect the impartiality of the information presented in this paper. “The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results”.
Figures
References
-
- Kalyanaraman B., Cheng G., Hardy M., Ouari O., Bennett B., Zielonka J. Teaching the basics of reactive oxygen species and their relevance to cancer biology: Mitochondrial reactive oxygen species detection, redox signaling, and targeted therapies. Redox Biol. 2018;15:347–362. doi: 10.1016/j.redox.2017.12.012. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

