Safety and effectiveness of granulocyte and monocyte adsorptive apheresis in patients with inflammatory bowel disease in special situations: a multicentre cohort study
- PMID: 31752695
- PMCID: PMC6873503
- DOI: 10.1186/s12876-019-1110-1
Safety and effectiveness of granulocyte and monocyte adsorptive apheresis in patients with inflammatory bowel disease in special situations: a multicentre cohort study
Abstract
Background: The available information on granulocyte and monocyte adsorptive apheresis (GMA) in patients with inflammatory bowel disease (IBD) under special situations remains unclear. We conducted a retrospective, multicentre cohort study to evaluate the safety and effectiveness of GMA in patients with IBD under special situations.
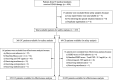
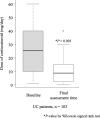
Methods: This study included patients with ulcerative colitis (UC) or Crohn's disease who had at least one special situation feature and who had received GMA between November 2013 and March 2017. The incidence of adverse events (AEs) was compared in relation to the special situation, and patient background factors related to an AE were identified. For patients with UC, clinical remission was defined as a partial Mayo score of ≤2.
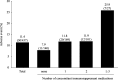
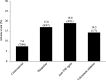
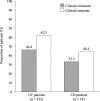
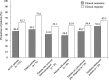
Results: A total of 437 patients were included in this study. The incidence of AEs among the elderly patients (11.2%) was similar in all patients (11.4%), whereas the incidences of AEs in patients on multiple immunosuppressant medications (15.2%), patients with anaemia (18.1%) and paediatric/adolescent patients (18.9%) were higher than that in all patients (11.4%). In multivariate analysis, anaemia and concomitant immunosuppressant medications were independently associated with the incidence of AEs. Clinical remission was achieved in 46.4% of the patients with UC.
Conclusions: The incidence of AEs in the elderly patients was not higher than that in all patients, whereas the incidence of AE was higher in patients with anaemia and those on multiple immunosuppressant medications than that in all patients. GMA is a safe treatment option in elderly patients with IBD.
Keywords: Anaemia; Crohn’s disease; Elderly patient; GMA; Granulocyte and monocyte adsorptive apheresis; Immunosuppressant; Inflammatory bowel disease; Paediatric patient; Special situation; Ulcerative colitis.
Conflict of interest statement
SM received consultancy fees from AbbVie G.K., Janssen Pharmaceutical K.K., Kyorin Pharmaceutical, Pfizer Japan Inc. and Takeda Pharmaceutical Co., Ltd.; lecture fees from AbbVie G.K., Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Corporation and Mochida Pharmaceutical Co., Ltd. and research grants from EA Pharma Co., Ltd., Janssen Pharmaceutical K.K, Takeda Pharmaceutical Co., Ltd. and Pfizer Japan Inc. HT received lecture fees from JIMRO Co., Ltd., AbbVie G.K., EA Pharma Co., Ltd., Mochida Pharmaceutical Co., Ltd., Kyorin Pharmaceutical and Mitsubishi Tanabe Pharma Corporation. TS and TO received research grants from JIMRO Co., Ltd. DS received research grants from JIMRO Co., Ltd. ST received research grants from EA Pharma Co., Ltd. MN served as a consultant for JIMRO Co., Ltd. SK and EH are personnel of JIMRO Co., Ltd.
Figures

References
-
- Benchimol EI, Bernstein CN, Bitton A, Carroll MW, Singh H, Otley AR, et al. Trends in epidemiology of pediatric inflammatory bowel disease in Canada: distributed network analysis of multiple population-based provincial health administrative databases. Am J Gastroenterol. 2017;112:1120–1134. doi: 10.1038/ajg.2017.97. - DOI - PMC - PubMed
-
- Jeuring SF, Van den Heuvel TR, Zeegers MP, Hameeteman WH, Romberg-Camps MJ, Oostenbrug LE, et al. Epidemiology and long-term outcome of inflammatory bowel disease diagnosed at elderly age-an increasing distinct entity. Inflamm Bowel Dis. 2016;22:1425–1434. doi: 10.1097/MIB.0000000000000738. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

