Melatonin is responsible for rice resistance to rice stripe virus infection through a nitric oxide-dependent pathway
- PMID: 31752902
- PMCID: PMC6869260
- DOI: 10.1186/s12985-019-1228-3
Melatonin is responsible for rice resistance to rice stripe virus infection through a nitric oxide-dependent pathway
Abstract
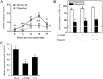
Rice stripe virus (RSV) causes one of the most important rice virus diseases of plants in East Asia. However, the molecular mechanisms controlling rice resistance to RSV infection are largely unknown. Recently, several studies presented a novel model that melatonin (MT) and nitric oxide (NO) participate in the plant-pathogen interaction in a synergetic manner. In this study, there was a difference in MT content between two rice varieties that correlated with one being susceptible and one being resistant to RSV, which suggested that MT is related to RSV resistance. In addition, a test with two NO biosynthesis inhibitors revealed that NO inhibitor were able to increase the disease incidence of RSV. A pharmacological experiment with exogenous MT and NO showed that increased MT and NO in the MT-pretreated plants led to lower disease incidences; however, only NO increased in a NO-releasing reagent [sodium nitroprusside (SNP)] pretreated plants. The expressions level of OsPR1b and OsWRKY 45 were significantly induced by MT and NO. These results suggest that rice resistance to RSV can be improved by increased MT through a NO-dependent pathway.
Keywords: Melatonin; Nitric oxide; Rice; Rice stripe virus.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Milne RG. The Filamentous Plant Viruses. In: Milne RG, editor. The Plant Viruses. New York: Plenum Press; 1988. pp. 297–329.
-
- Sun F, Yuan X, Zhou T, Fan Y, Zhou Y. Arabidopsis is susceptible to Rice stripe virus infections. J Phytopathol. 2011;159:767–772. doi: 10.1111/j.1439-0434.2011.01840.x. - DOI
-
- Toriyama S. Rice stripe virus: prototype of a new group of viruses that replicate in plants and insects. Microbiol Sci. 1986;3:347. - PubMed
-
- Yamashit S, Doi Y, Yora K. Intracellular appearance of rice stripe virus. Ann Phytopathol Soc Jpn. 1985;51:637–641. doi: 10.3186/jjphytopath.51.637. - DOI
-
- Lerner A, Case J, Takahashi Y, Lee TH. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J Amer Chem Soc. 1958;80:10. doi: 10.1021/ja01543a060. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

