Distinct migratory pattern of naive and effector T cells through the blood-CSF barrier following Echovirus 30 infection
- PMID: 31752904
- PMCID: PMC6868812
- DOI: 10.1186/s12974-019-1626-x
Distinct migratory pattern of naive and effector T cells through the blood-CSF barrier following Echovirus 30 infection
Abstract
Background: Echovirus 30 (E-30) is one of the most frequently isolated pathogens in aseptic meningitis worldwide. To gain access to the central nervous system (CNS), E-30 and immune cells have to cross one of the two main barriers of the CNS, the epithelial blood-cerebrospinal fluid barrier (BCSFB) or the endothelial blood-brain barrier (BBB). In an in vitro model of the BCSFB, it has been shown that E-30 can infect human immortalized brain choroid plexus papilloma (HIBCPP) cells.
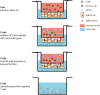
Methods: In this study we investigated the migration of different T cell subpopulations, naive and effector T cells, through HIBCPP cells during E-30 infection. Effects of E-30 infection and the migration process were evaluated via immunofluorescence and flow cytometry analysis, as well as transepithelial resistance and dextran flux measurement.
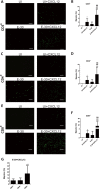
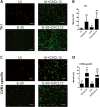
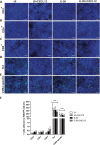
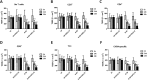
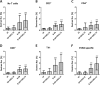
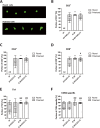
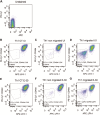
Results: Th1 effector cells and enterovirus-specific effector T cells migrated through HIBCPP cells more efficiently than naive CD4+ T cells following E-30 infection of HIBCPP cells. Among the different naive T cell populations, CD8+ T cells crossed the E-30-infected HIBCPP cell layer in a significantly higher number than CD4+ T cells. A large amount of effector T cells also remained attached to the basolateral side of the HIBCPP cells compared with naive T cells. Analysis of HIBCPP barrier function showed significant alteration after E-30 infection and trans- as well as paracellular migration of T cells independent of the respective subpopulation. Morphologic analysis of migrating T cells revealed that a polarized phenotype was induced by the chemokine CXCL12, but reversed to a round phenotype after E-30 infection. Further characterization of migrating Th1 effector cells revealed a downregulation of surface adhesion proteins such as LFA-1 PSGL-1, CD44, and CD49d.
Conclusion: Taken together these results suggest that naive CD8+ and Th1 effector cells are highly efficient to migrate through the BCSFB in an inflammatory environment. The T cell phenotype is modified during the migration process through HIBCPP cells.
Keywords: Blood–cerebrospinal fluid barrier; Effector T cells; Enterovirus; Meningitis; Naive T cells; T cell migration.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Chemotaxis of T-cells after infection of human choroid plexus papilloma cells with Echovirus 30 in an in vitro model of the blood-cerebrospinal fluid barrier.Virus Res. 2012 Dec;170(1-2):66-74. doi: 10.1016/j.virusres.2012.08.019. Epub 2012 Sep 19. Virus Res. 2012. PMID: 23000117
-
Polar Infection of Echovirus-30 Causes Differential Barrier Affection and Gene Regulation at the Blood-Cerebrospinal Fluid Barrier.Int J Mol Sci. 2020 Aug 29;21(17):6268. doi: 10.3390/ijms21176268. Int J Mol Sci. 2020. PMID: 32872518 Free PMC article.
-
Strain-dependent effects of clinical echovirus 30 outbreak isolates at the blood-CSF barrier.J Neuroinflammation. 2018 Feb 20;15(1):50. doi: 10.1186/s12974-018-1061-4. J Neuroinflammation. 2018. PMID: 29463289 Free PMC article.
-
Immune surveillance of the human central nervous system (CNS): different migration pathways of immune cells through the blood-brain barrier and blood-cerebrospinal fluid barrier in healthy persons.Cytometry A. 2006 Mar;69(3):147-51. doi: 10.1002/cyto.a.20225. Cytometry A. 2006. PMID: 16479603 Review.
-
A new in vitro model for blood-cerebrospinal fluid barrier transport studies: an immortalized choroid plexus epithelial cell line derived from the tsA58 SV40 large T-antigen gene transgenic rat.Adv Drug Deliv Rev. 2004 Oct 14;56(12):1875-85. doi: 10.1016/j.addr.2004.07.013. Adv Drug Deliv Rev. 2004. PMID: 15381338 Review.
Cited by
-
Human CD4+ T cell subsets differ in their abilities to cross endothelial and epithelial brain barriers in vitro.Fluids Barriers CNS. 2020 Feb 3;17(1):3. doi: 10.1186/s12987-019-0165-2. Fluids Barriers CNS. 2020. PMID: 32008573 Free PMC article.
-
Delivery strategies for cell-based therapies in the brain: overcoming multiple barriers.Drug Deliv Transl Res. 2021 Dec;11(6):2448-2467. doi: 10.1007/s13346-021-01079-1. Epub 2021 Oct 30. Drug Deliv Transl Res. 2021. PMID: 34718958 Free PMC article.
-
Localization of ZIP14 and ZIP8 in HIBCPP Cells.Brain Sci. 2020 Aug 8;10(8):534. doi: 10.3390/brainsci10080534. Brain Sci. 2020. PMID: 32784388 Free PMC article.
-
The hypervirulent Group B Streptococcus HvgA adhesin promotes central nervous system invasion through transcellular crossing of the choroid plexus.Fluids Barriers CNS. 2024 Aug 16;21(1):66. doi: 10.1186/s12987-024-00564-2. Fluids Barriers CNS. 2024. PMID: 39152442 Free PMC article.
-
The colibactin-producing Escherichia coli alters the tumor microenvironment to immunosuppressive lipid overload facilitating colorectal cancer progression and chemoresistance.Gut Microbes. 2024 Jan-Dec;16(1):2320291. doi: 10.1080/19490976.2024.2320291. Epub 2024 Feb 28. Gut Microbes. 2024. PMID: 38417029 Free PMC article.
References
-
- Holmes CW, Koo SS, Osman H, Wilson S, Xerry J, Gallimore CI, Allen DJ, Tang JW. Predominance of enterovirus B and echovirus 30 as cause of viral meningitis in a UK population. J Clin Virol. 2016;81:90–93. - PubMed
-
- Bouslama L, Gharbi J, Aouni M. Analysis of the genetic and the corresponding antigenic variability of the VP1 3′ end of ECHO virus type 11 and ECHO virus type 30. Virus Genes. 2006;33:205–212. - PubMed
-
- Kieslich M, Acconci D, Berger A, Jarisch A, Bohles H, Bollinger M, Jacobi G, Hernaiz Driever P. Diagnosis and outcome of neurotropic enterovirus infections in childhood. Klin Padiatr. 2002;214:327–331. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

