Reduction of the long-term use of proton pump inhibitors by a patient-oriented electronic decision support tool (arriba-PPI): study protocol for a randomized controlled trial
- PMID: 31752978
- PMCID: PMC6868794
- DOI: 10.1186/s13063-019-3728-2
Reduction of the long-term use of proton pump inhibitors by a patient-oriented electronic decision support tool (arriba-PPI): study protocol for a randomized controlled trial
Abstract
Background: Proton pump inhibitors (PPIs) are increasingly being prescribed, although long-term use is associated with multiple side effects. Therefore, an electronic decision support tool with the aim of reducing the long-term use of PPIs in a shared decision-making process between general practitioners (GPs) and their patients has been developed. The developed tool is a module that can be added to the so-called arriba decision support tool, which is already used by GPs in Germany in routine care. In this large-scale cluster-randomized controlled trial we evaluate the effectiveness of this arriba-PPI tool.
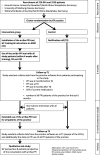
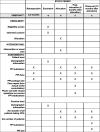
Methods: The arriba-PPI tool is an electronic decision support system that supports shared decision-making and evidence-based decisions around the long-term use of PPIs at the point of care. The tool will be evaluated in a cluster-randomized controlled trial involving 210 GP practices and 3150 patients in Germany. GP practices will be asked to recruit 20 patients aged ≥ 18 years regularly taking PPIs for ≥ 6 months. After completion of patient recruitment, each GP practice with enrolled patients will be cluster-randomized. Intervention GP practices will get access to the software arriba-PPI, whereas control GPs will treat their patients as usual. After an observation period of six months, GP practices will be compared regarding the reduction of cumulated defined daily doses of PPI prescriptions per patient.
Discussion: Our principal hypothesis is that the application of the arriba-PPI tool can reduce PPI prescribing in primary care by at least 15% compared to conventional strategies used by GPs. A positive result implies the implementation of the arriba-PPI tool in routine care.
Trial registration: German Clinical Trials Register, DRKS00016364. Registered on 31 January 2019.
Keywords: Computerized clinical decision support system; Deprescribing; Evidence-based medicine; General practitioner; Proton pump inhibitors.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Schwabe U, Paffrath D, editors. Arzneiverordnungs-Report 2016: Aktuelle Daten, Kosten, Trends und Kommentare. Berlin: Springer Berlin Heidelberg; 2016.
-
- Schwabe U, Paffrath D, Ludwig W-D, Klauber J, editors. Arzneiverordnungs-Report 2019. 1. Berlin: Springer Berlin; Springer; 2019.
-
- Sigterman KE, van Pinxteren B, Bonis PA, Lau J, Numans ME. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev. 2013;96:2849. doi: 10.1002/14651858.CD002095.pub5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources