Nutrition therapy and critical illness: practical guidance for the ICU, post-ICU, and long-term convalescence phases
- PMID: 31752979
- PMCID: PMC6873712
- DOI: 10.1186/s13054-019-2657-5
Nutrition therapy and critical illness: practical guidance for the ICU, post-ICU, and long-term convalescence phases
Abstract
Background: Although mortality due to critical illness has fallen over decades, the number of patients with long-term functional disabilities has increased, leading to impaired quality of life and significant healthcare costs. As an essential part of the multimodal interventions available to improve outcome of critical illness, optimal nutrition therapy should be provided during critical illness, after ICU discharge, and following hospital discharge.
Methods: This narrative review summarizes the latest scientific insights and guidelines on ICU nutrition delivery. Practical guidance is given to provide optimal nutrition therapy during the three phases of the patient journey.
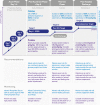
Results: Based on recent literature and guidelines, gradual progression to caloric and protein targets during the initial phase of ICU stay is recommended. After this phase, full caloric dose can be provided, preferably based on indirect calorimetry. Phosphate should be monitored to detect refeeding hypophosphatemia, and when occurring, caloric restriction should be instituted. For proteins, at least 1.3 g of proteins/kg/day should be targeted after the initial phase. During the chronic ICU phase, and after ICU discharge, higher protein/caloric targets should be provided preferably combined with exercise. After ICU discharge, achieving protein targets is more difficult than reaching caloric goals, in particular after removal of the feeding tube. After hospital discharge, probably very high-dose protein and calorie feeding for prolonged duration is necessary to optimize the outcome. High-protein oral nutrition supplements are likely essential in this period. Several pharmacological options are available to combine with nutrition therapy to enhance the anabolic response and stimulate muscle protein synthesis.
Conclusions: During and after ICU care, optimal nutrition therapy is essential to improve the long-term outcome to reduce the likelihood of the patient to becoming a "victim" of critical illness. Frequently, nutrition targets are not achieved in any phase of recovery. Personalized nutrition therapy, while respecting different targets during the phases of the patient journey after critical illness, should be prescribed and monitored.
Keywords: Autophagy; Calories; Enteral feeding; Exercise; Micronutrients; Mitochondrial dysfunction; Oral nutrition supplements; Overfeeding; Parenteral feeding; Protein; Refeeding syndrome; Underfeeding.
Conflict of interest statement
Dr. Van Zanten reported having received honoraria for advisory board meetings, lectures, research, and travel expenses from Abbott, Baxter, BBraun, Cardinal Health, Danone-Nutricia, Fresenius Kabi, Mermaid, Lyric, and Nestlé-Novartis. Inclusion fees for patients in clinical trials were paid to the local ICU research foundation.
Dr. De Waele reported having received grant funding from the Belgian Government of Health and grant funding and honoraria for advisory board meetings, lectures, and travel expenses from Baxter, Fresenius Kabi, BBraun, Cardinal Health, and Danone-Nutricia.
Dr. Wischmeyer reported receiving grant funding related to this work from the National Institutes of Health, Canadian Institutes of Health Research, Abbott, Baxter, Fresenius, and Takeda. Dr. Wischmeyer has served as a consultant to Abbott, Fresenius, Baxter, Cardinal Health, Nutricia, and Takeda for research related to this work. Dr. Wischmeyer has received unrestricted gift donation for nutrition research from Musclesound and Cosmed. Dr. Wischmeyer has received honoraria or travel expenses for CME lectures on improving nutrition care from Abbott, Baxter, and Danone-Nutricia.
Figures
References
-
- Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H, Zawistowski C, Bemis-Dougherty A, Berney SC, Bienvenu OJ, Brady SL, Brodsky MB, Denehy L, Elliott D, Flatley C, Harabin AL, Jones C, Louis D, Meltzer W, Muldoon SR, Palmer JB, Perme C, Robinson M, Schmidt DM, Scruth E, Spill GR, Storey CP, Render M, Votto J, Harvey MA. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40(2):502–509. doi: 10.1097/CCM.0b013e318232da75. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

