Functional Diversification of ER Stress Responses in Arabidopsis
- PMID: 31753702
- PMCID: PMC6980780
- DOI: 10.1016/j.tibs.2019.10.008
Functional Diversification of ER Stress Responses in Arabidopsis
Abstract
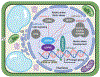
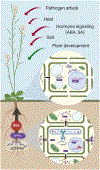
The endoplasmic reticulum (ER) is responsible for the synthesis of one-third of the cellular proteome and is constantly challenged by physiological and environmental situations that can perturb its homeostasis and lead to the accumulation of misfolded secretory proteins, a condition referred to as ER stress. In response, the ER evokes a set of intracellular signaling processes, collectively known as the unfolded protein response (UPR), which are designed to restore biosynthetic capacity of the ER. As single-cell organisms evolved into multicellular life, the UPR complexity has increased to suit their growth and development. In this review, we discuss recent advances in the understanding of the UPR, emphasizing conserved UPR elements between plants and metazoans and highlighting unique plant-specific features.
Keywords: Arabidopsis thaliana.; ER stress; unfolded protein response (UPR).
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures

References
-
- Walter P and Ron D (2011) The Unfolded Protein Response: From Stress Pathway to Homeostatic Regulation. Science 334 (6059), 1081–1086. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

