Ramucirumab plus docetaxel versus placebo plus docetaxel in patients with locally advanced or metastatic urothelial carcinoma after platinum-based therapy (RANGE): overall survival and updated results of a randomised, double-blind, phase 3 trial
- PMID: 31753727
- PMCID: PMC6946880
- DOI: 10.1016/S1470-2045(19)30668-0
Ramucirumab plus docetaxel versus placebo plus docetaxel in patients with locally advanced or metastatic urothelial carcinoma after platinum-based therapy (RANGE): overall survival and updated results of a randomised, double-blind, phase 3 trial
Abstract
Background: Ramucirumab-an IgG1 vascular endothelial growth factor receptor 2 antagonist-plus docetaxel was previously reported to improve progression-free survival in platinum-refractory, advanced urothelial carcinoma. Here, we report the secondary endpoint of overall survival results for the RANGE trial.
Methods: We did a randomised, double-blind, phase 3 trial in patients with advanced or metastatic urothelial carcinoma who progressed during or after platinum-based chemotherapy. Patients were enrolled from 124 investigative sites (hospitals, clinics, and academic centres) in 23 countries. Previous treatment with one immune checkpoint inhibitor was permitted. Patients were randomly assigned (1:1) using an interactive web response system to receive intravenous ramucirumab 10 mg/kg or placebo 10 mg/kg volume equivalent followed by intravenous docetaxel 75 mg/m2 (60 mg/m2 in Korea, Taiwan, and Japan) on day 1 of a 21-day cycle. Treatment continued until disease progression, unacceptable toxicity, or other discontinuation criteria were met. Randomisation was stratified by geographical region, Eastern Cooperative Oncology Group performance status at baseline, and visceral metastasis. Progression-free survival (the primary endpoint) and overall survival (a key secondary endpoint) were assessed in the intention-to-treat population. The study is registered with ClinicalTrials.gov, NCT02426125; patient enrolment is complete and the last patient on treatment is being followed up for safety issues.
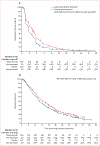
Findings: Between July 20, 2015, and April 4, 2017, 530 patients were randomly allocated to ramucirumab plus docetaxel (n=263) or placebo plus docetaxel (n=267) and comprised the intention-to-treat population. At database lock (March 21, 2018) for the final overall survival analysis, median follow-up was 7·4 months (IQR 3·5-13·9). In our sensitivity analysis of investigator-assessed progression-free survival at the overall survival database lock, median progression-free survival remained significantly improved with ramucirumab compared with placebo (4·1 months [95% CI 3·3-4·8] vs 2·8 months [2·6-2·9]; HR 0·696 [95% CI 0·573-0·845]; p=0·0002). Median overall survival was 9·4 months (95% CI 7·9-11·4) in the ramucirumab group versus 7·9 months (7·0-9·3) in the placebo group (stratified HR 0·887 [95% CI 0·724-1·086]; p=0·25). Grade 3 or worse treatment-related treatment-emergent adverse events in 5% or more of patients and with an incidence more than 2% higher with ramucirumab than with placebo were febrile neutropenia (24 [9%] of 258 patients in the ramucirumab group vs 16 [6%] of 265 patients in the placebo group) and neutropenia (17 [7%] of 258 vs six [2%] of 265). Serious adverse events were similar between groups (112 [43%] of 258 patients in the ramucirumab group vs 107 [40%] of 265 patients in the placebo group). Adverse events related to study treatment and leading to death occurred in eight (3%) patients in the ramucirumab group versus five (2%) patients in the placebo group.
Interpretation: Additional follow-up supports that ramucirumab plus docetaxel significantly improves progression-free survival, without a significant improvement in overall survival, for patients with platinum-refractory advanced urothelial carcinoma. Clinically meaningful benefit might be restricted in an unselected population.
Funding: Eli Lilly and Company.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests
DPP reports grants from Eli Lilly and Company, during the conduct of the study; and consultant fees from Ada Cap, Amgen, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Clovis, Eli Lilly, Exelixis, Incyte, Janssen, Pfizer, Pharmacyclics, Roche Laboratories, Seattle Genetics, and Urogen, grants from Ada Cap, Astellas, AstraZeneca, Bayer, Bristol-Myers Squibb, Clovis, Eli Lilly, Endocyte, Genentech, Innocrin, MedImmune, Merck, Novartis, Pfizer, Progenics, Roche Laboratories, Sanofi Aventis, and Seattle Genetics, and ownership interest or investments in Bellicum and Tyme, outside the submitted work. RdW reports personal fees from Eli Lilly and Company, during the conduct of the study; and grants (paid to institution) from Sanofi and personal fees from Merck, Roche, Bayer, Janssen, Clovis, and Sanofi, outside the submitted work. KNC reports institutional funding from Eli Lilly and Company, during the conduct of the study. AD reports travel reimbursements from Eli Lilly and Company, consulting fees from Bristol-Myers Squibb, travel and consulting fees from AstraZeneca, and equity in Kynan Pharma, Allogene, and Urogen, outside the submitted work. CNS reports personal fees from Eli Lilly and Company, Bristol-Myers Squibb, Merck/Pfizer, and Clovis, outside the submitted work. SAH reports personal fees from Roche, Merck, AstraZeneca, Pierre Fabre, Sotio, Pfizer, Janssen, and Bayer, outside the submitted work. AF reports honoraria from AstraZeneca, Merck Sharp & Dohme, and Roche, outside the submitted work. AB reports personal fees from AstraZeneca and Bristol-Myers Squibb, and grants and personal fees from Roche, outside the submitted work. EYY reports grants and personal fees from Eli Lilly, during the conduct of the study; and grants and personal fees from Agensys, Astellas, Bayer, Dendreon, Genentech/Roche, Merck, and Seattle Genetics and personal fees from AstraZeneca, Churchill Pharmaceuticals, EMD Serono, Ferring, Janssen, Medivation, Sanofi, Tolmar, Tokai, QED, Amgen, Pharmacyclics, and InCyte, outside the submitted work. MSvdH reports grants (paid to institution) from Roche and AstraZeneca and consultation and grant support (both to institute) from Bristol-Myers Squibb, outside the submitted work.
NM reports research grants from Janssen, AstraZeneca, Merck Sharp & Dohme, Bayer, Lilly, Roche, and Taiho, outside the submitted work. AN reports grants, personal fees, and non-financial support from Roche, grants and personal fees from Merck and AstraZeneca, and personal fees from Seattle Genetics and Bayer, during the conduct of the study. JB reports grants from Eli Lilly and Company, during the conduct of the study; and personal fees from AstraZeneca, Astellas, and Eusa Pharma and grants and personal fees from Bristol-Myers Squibb, Eisai, Ipsen, Merck Sharp & Dohme, Novartis, Roche, and Pfizer, and personal fees from Eusa Pharma, outside the submitted work. J-LL reports grants, personal fees, and advisory board fees from Pfizer Korea and Ipsen Korea, personal fees and advisory board fees from Janssen, Astellas Korea, Bristol-Myers Squibb Korea, and Sanofi Aventis, and personal fees from Novartis Korea, outside the submitted work. MT reports personal fees from Astellas, Sanofi, Bayer, and Janssen, outside the submitted work. AP reports consultant fees from Merck Sharp & Dohme, Bayer, Bristol-Myers Squibb, Janssen, Astellas, Novartis, Roche, AstraZeneca, and Pfizer, outside the submitted work. XGdM reports personal fees from Pfizer, Bristol-Myers Squibb, Eli Lilly and Company, Pharmamar, Novartis, Ipsen, and Roche, outside the submitted work. AR-V reports grants from Takeda, grants and personal fees from Merck Sharp and Dohme, and grants and personal fees from Pfizer, Janssen, Astellas, Roche, Bristol-Myers Squibb, Clovis, Bayer, and Sanofi-Aventis, outside the submitted work. STT reports institutional research support from Eli Lilly during the conduct of the study, and prior honoraria from Sanofi; and consulting and advisory roles for Medivation, Astellas Pharma, Dendreon, Janssen, Bayer, Genentech, Sanofi, Endocyte, Immunomedics, Karyopharm Therapeutics, Abbvie, Tolmark, QED, institutional research support from Janssen, Astellas Pharma, Progenics, Millennium, Amgen, Bristol-Myers Squibb, Dendreon, Rexahn Pharmaceuticals, Bayer, Genentech, Newlink Genetics, Inovio Pharmaceuticals, AstraZeneca, Immunomedics, Novartis, AVEO, Boehringer Ingelheim, Merck Sharp & Dohme, Stem CentRx, Karyopharm Therapeutics, Abbvie, Medivation, Endocyte, Exelixis, and Clovis Oncology, and travel, accommodations, and expenses received from Sanofi and Amgen, outside the submitted work. UV reports personal fees (honoraria) and other (consulting, advisory, or speaker bureau payments) from Bayer and Sanofi, grants, personal fees, and consulting, advisory, or speaker bureau payments from Bristol-Myers Squibb, Exelixis, and Pfizer, and grants and consulting or advisory role payments from Astellas Pharma, outside the submitted work. JBA-C reports personal fees from Bristol-Myers Squibb, Sanofi, Janssen, and EMD Serono outside the submitted work. OH and FR were employees of Eli Lilly and Company during the conduct of the study and are shareholders at Eli Lilly and Company. AML, SW, RRH, AHZ, and KMB-M are employees and shareholders at Eli Lilly and Company. RAW is an employee, shareholder, and has patents pending at Eli Lilly and Company. TP reports strategic or advisory fees and grants from Roche, strategic or advisory and consultant or honoraria fees from Pfizer, Bristol-Myers Squibb, and AstraZeneca, and consultant or honoraria fees from Exelexis, Incyte, Ipsen, Merck, and Seattle Genetics, all outside the submitted work. All other authors declare no competing interests.
Figures


Comment in
-
Critical treatment choices for patients with platinum-refractory urothelial carcinoma.Lancet Oncol. 2020 Jan;21(1):11-13. doi: 10.1016/S1470-2045(19)30661-8. Epub 2019 Nov 18. Lancet Oncol. 2020. PMID: 31753728 No abstract available.
References
-
- Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018;391: 748–57. - PubMed
-
- Raggi D, Miceli R, Sonpavde G, et al. Second-line single-agent versus doublet chemotherapy as salvage therapy for metastatic urothelial cancer: a systematic review and meta-analysis. Ann Oncol 2016; 27: 49–61. - PubMed
-
- Bellmunt J, Théodore C, Demkov T, et al. Phase 3 trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol 2009; 27: 4454–61. - PubMed
-
- Vaughn DJ, Srinivas S, Stadler WM, et al. Vinflunine in platinum-pretreated patients with locally advanced or metastatic urothelial carcinoma: results of a large phase 2 study. Cancer 2009; 115: 4110–17. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

