Changing of the guard: How the Lyme disease spirochete subverts the host immune response
- PMID: 31753921
- PMCID: PMC6956529
- DOI: 10.1074/jbc.REV119.008583
Changing of the guard: How the Lyme disease spirochete subverts the host immune response
Abstract
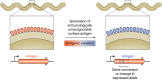
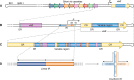
Lyme disease, also known as Lyme borreliosis, is the most common tick-transmitted disease in the Northern Hemisphere. The disease is caused by the bacterial spirochete Borrelia burgdorferi and other related Borrelia species. One of the many fascinating features of this unique pathogen is an elaborate system for antigenic variation, whereby the sequence of the surface-bound lipoprotein VlsE is continually modified through segmental gene conversion events. This perpetual changing of the guard allows the pathogen to remain one step ahead of the acquired immune response, enabling persistent infection. Accordingly, the vls locus is the most evolutionarily diverse genetic element in Lyme disease-causing borreliae. Small stretches of information are transferred from a series of silent cassettes in the vls locus to generate an expressed mosaic vlsE gene version that contains genetic information from several different silent cassettes, resulting in ∼1040 possible vlsE sequences. Yet, despite its extreme evolutionary flexibility, the locus has rigidly conserved structural features. These include a telomeric location of the vlsE gene, an inverse orientation of vlsE and the silent cassettes, the presence of nearly perfect inverted repeats of ∼100 bp near the 5' end of vlsE, and an exceedingly high concentration of G runs in vlsE and the silent cassettes. We discuss the possible roles of these evolutionarily conserved features, highlight recent findings from several studies that have used next-generation DNA sequencing to unravel the switching process, and review advances in the development of a mini-vls system for genetic manipulation of the locus.
Keywords: Borrelia; DNA mismatch repair; DNA recombination; G-quadruplex; Lyme disease; antigenic variation; bacterial genetics; bacterial pathogenesis; gene conversion; genetic polymorphism; infectious disease.
© 2020 Chaconas et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

