XPO1 regulates erythroid differentiation and is a new target for the treatment of β-thalassemia
- PMID: 33054049
- PMCID: PMC7556489
- DOI: 10.3324/haematol.2018.210054
XPO1 regulates erythroid differentiation and is a new target for the treatment of β-thalassemia
Abstract
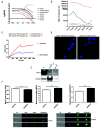
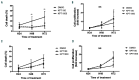
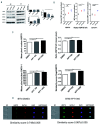
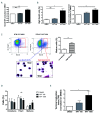
β-thalassemia major (β-TM) is an inherited hemoglobinopathy caused by a quantitative defect in the synthesis of β-globin chains of hemoglobin, leading to the accumulation of free a-globin chains that aggregate and cause ineffective erythropoiesis. We have previously demonstrated that terminal erythroid maturation requires a transient activation of caspase-3 and that the chaperone Heat Shock Protein 70 (HSP70) accumulates in the nucleus to protect GATA-1 transcription factor from caspase-3 cleavage. This nuclear accumulation of HSP70 is inhibited in human β-TM erythroblasts due to HSP70 sequestration in the cytoplasm by free a-globin chains, resulting in maturation arrest and apoptosis. Likewise, terminal maturation can be restored by transduction of a nuclear-targeted HSP70 mutant. Here we demonstrate that in normal erythroid progenitors, HSP70 localization is regulated by the exportin-1 (XPO1), and that treatment of β-thalassemic erythroblasts with an XPO1 inhibitor increased the amount of nuclear HSP70, rescued GATA-1 expression and improved terminal differentiation, thus representing a new therapeutic option to ameliorate ineffective erythropoiesis of β-TM.
Figures
Comment in
-
Novel use for selective inhibitors of nuclear export in β-thalassemia: block of HSP70 export from the nucleus via exportin Xpo1 improves ineffective erythropoiesis.Haematologica. 2020 Sep 1;105(9):2188-2189. doi: 10.3324/haematol.2020.254474. Haematologica. 2020. PMID: 33054040 Free PMC article. No abstract available.
References
-
- Ribeil J-A, Zermati Y, Vandekerckhove J, et al. Hsp70 regulates erythropoiesis by preventing caspase-3-mediated cleavage of GATA-1. Nature. 2007;445(7123):102-105. - PubMed
-
- Arlet J-B, Ribeil J-A, Guillem F, et al. HSP70 sequestration by free a-globin promotes ineffective erythropoiesis in β-thalassaemia. Nature. 2014;514(7521):242-246. - PubMed
-
- Kudo N, Wolff B, Sekimoto T, et al. Leptomycin B inhibition of signal-mediated nuclear export by direct binding to CRM1. Exp Cell Res. 1998;242(2):540-547. - PubMed
-
- Nishi K, Yoshida M, Fujiwara D, Nishikawa M, Horinouchi S, Beppu T. Leptomycin B targets a regulatory cascade of crm1, a fission yeast nuclear protein, involved in control of higher order chromosome structure and gene expression. J Biol Chem. 1994; 269(9):6320-6324. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

