Calcium signalling in mammalian cell lines expressing wild type and mutant human α1-Antitrypsin
- PMID: 31754242
- PMCID: PMC6872872
- DOI: 10.1038/s41598-019-53535-1
Calcium signalling in mammalian cell lines expressing wild type and mutant human α1-Antitrypsin
Abstract
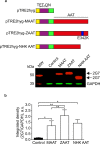
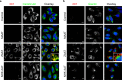
A possible role for calcium signalling in the autosomal dominant form of dementia, familial encephalopathy with neuroserpin inclusion bodies (FENIB), has been proposed, which may point towards a mechanism by which cells could sense and respond to the accumulation of mutant serpin polymers in the endoplasmic reticulum (ER). We therefore explored possible defects in Ca2+-signalling, which may contribute to the pathology associated with another serpinopathy, α1-antitrypsin (AAT) deficiency. Using CHO K1 cell lines stably expressing a wild type human AAT (MAAT) and a disease-causing polymer-forming variant (ZAAT) and the truncated variant (NHK AAT), we measured basal intracellular free Ca2+, its responses to thapsigargin (TG), an ER Ca2+-ATPase blocker, and store-operated Ca2+-entry (SOCE). Our fura2 based Ca2+ measurements detected no differences between these 3 parameters in cell lines expressing MAAT and cell lines expressing ZAAT and NHK AAT mutants. Thus, in our cell-based models of α1-antitrypsin (AAT) deficiency, unlike the case for FENIB, we were unable to detect defects in calcium signalling.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Silverman GA, et al. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. Journal of Biological Chemistry. 2001;276:33293–33296. doi: 10.1074/jbc.R100016200. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

