Micheliolide Inhibits Liver Cancer Cell Growth Via Inducing Apoptosis And Perturbing Actin Cytoskeleton
- PMID: 31754310
- PMCID: PMC6825479
- DOI: 10.2147/CMAR.S216870
Micheliolide Inhibits Liver Cancer Cell Growth Via Inducing Apoptosis And Perturbing Actin Cytoskeleton
Abstract
Purpose: Micheliolide (MCL) is an effector compound of the flower which has been traditionally used to treat inflammation and cancer patients in oriental medicine. MCL has killing effects on several cancer and immune cells by modulating apoptosis, cell cycle, and metabolism. However, the detail of the mechanisms of anti-cancer activity remains to be elucidated and the effect on liver cancer cells is unknown.
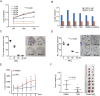
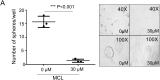
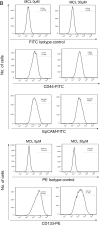
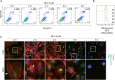
Methods: Cell proliferation was determined by CCK8 and clone formation assay. The xenograft liver cancer model formed by injecting Huh7 cells into NUDE mice was used to evaluate the effects of MCL on liver cancer cells in vivo. We evaluated the stemness of cells with spheroid formation assay and flow cytometry assay. The apoptosis was determined by Annexin V assay. F-actin staining and ROS were performed to detect the impairment of the F-actin cytoskeleton and mitochondria.
Results: Here, we first show that MCL inhibits liver cancer cells both in vivo and in vitro by triggering apoptosis which was reduced by anti-oxidant, but not cell-cycle arrest. In addition, MCL induces mitochondrial ROS and caspase-3 activation. Also, we found that the aggregation of mitochondria and the perturbation of F-actin fibers in the MCL-treated liver cancer cells coincidently occurred before the induction of apoptosis and mitochondrial ROS.
Conclusion: These results suggest that F-actin perturbation is involved in impaired mitochondria and apoptosis. Therefore, MCL can be a potent therapeutic reagent for liver cancer, primarily targeting the actin cytoskeleton.
Keywords: Micheliolide; ROS; actin cytoskeleton; apoptosis; liver cancer.
© 2019 Yu et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials

