Proliferative regulation of alveolar epithelial type 2 progenitor cells by human Scnn1d gene
- PMID: 31754387
- PMCID: PMC6857051
- DOI: 10.7150/thno.37023
Proliferative regulation of alveolar epithelial type 2 progenitor cells by human Scnn1d gene
Abstract
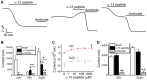
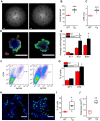
Lung epithelial sodium channel (ENaC) encoded by Scnn1 genes is essential for maintaining transepithelial salt and fluid homeostasis in the airway and the lung. Compared to α, β, and γ subunits, the role of respiratory δ-ENaC has not been studied in vivo due to the lack of animal models. Methods: We characterized full-length human δ802-ENaC expressed in both Xenopus oocytes and humanized transgenic mice. AT2 proliferation and differentiation in 3D organoids were analysed with FACS and a confocal microscope. Both two-electrode voltage clamp and Ussing chamber systems were applied to digitize δ802-ENaC channel activity. Immunoblotting was utilized to analyse δ802-ENaC protein. Transcripts of individual ENaC subunits in human lung tissues were quantitated with qPCR. Results: The results indicate that δ802-ENaC functions as an amiloride-inhibitable Na+ channel. Inhibitory peptide α-13 distinguishes δ802- from α-type ENaC channels. Modified proteolysis of γ-ENaC by plasmin and aprotinin did not alter the inhibition of amiloride and α-13 peptide. Expression of δ802-ENaC at the apical membrane of respiratory epithelium was detected with biophysical features similar to those of heterologously expressed channels in oocytes. δ802-ENaC regulated alveologenesis through facilitating the proliferation of alveolar type 2 epithelial cells. Conclusion: The humanized mouse line conditionally expressing human δ802-ENaC is a novel model for studying the expression and function of this protein in vivo .
Keywords: alveolar type epithelial cells; epithelial sodium channels; humanized transgenic mouse line; self-renewal.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
-
- Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD. et al. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994;367:463–7. - PubMed
-
- Waldmann R, Champigny G, Bassilana F, Voilley N, Lazdunski M. Molecular cloning and functional expression of a novel amiloride-sensitive Na+ channel. J Biol Chem. 1995;270:27411–4. - PubMed
-
- Lingueglia E, Voilley N, Waldmann R, Lazdunski M, Barbry P. Expression cloning of an epithelial amiloride-sensitive Na+ channel. A new channel type with homologies to Caenorhabditis elegans degenerins. FEBS Lett. 1993;318:95–9. - PubMed
-
- Ji HL, Benos DJ. Degenerin sites mediate proton activation of deltabetagamma-epithelial sodium channel. J Biol Chem. 2004;279:26939–47. - PubMed
-
- Chalfie M, Wolinsky E. The identification and suppression of inherited neurodegeneration in Caenorhabditis elegans. Nature. 1990;345:410–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

