A 25 micron-thin microscope for imaging upconverting nanoparticles with NIR-I and NIR-II illumination
- PMID: 31754393
- PMCID: PMC6857055
- DOI: 10.7150/thno.37672
A 25 micron-thin microscope for imaging upconverting nanoparticles with NIR-I and NIR-II illumination
Abstract
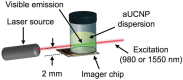
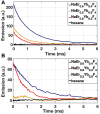
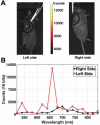
Rationale: Intraoperative visualization in small surgical cavities and hard-to-access areas are essential requirements for modern, minimally invasive surgeries and demand significant miniaturization. However, current optical imagers require multiple hard-to-miniaturize components including lenses, filters and optical fibers. These components restrict both the form-factor and maneuverability of these imagers, and imagers largely remain stand-alone devices with centimeter-scale dimensions. Methods: We have engineered INSITE (Immunotargeted Nanoparticle Single-Chip Imaging Technology), which integrates the unique optical properties of lanthanide-based alloyed upconverting nanoparticles (aUCNPs) with the time-resolved imaging of a 25-micron thin CMOS-based (complementary metal oxide semiconductor) imager. We have synthesized core/shell aUCNPs of different compositions and imaged their visible emission with INSITE under either NIR-I and NIR-II photoexcitation. We characterized aUCNP imaging with INSITE across both varying aUCNP composition and 980 nm and 1550 nm excitation wavelengths. To demonstrate clinical experimental validity, we also conducted an intratumoral injection into LNCaP prostate tumors in a male nude mouse that was subsequently excised and imaged with INSITE. Results: Under the low illumination fluences compatible with live animal imaging, we measure aUCNP radiative lifetimes of 600 μs - 1.3 ms, which provides strong signal for time-resolved INSITE imaging. Core/shell NaEr0.6Yb0.4F4 aUCNPs show the highest INSITE signal when illuminated at either 980 nm or 1550 nm, with signal from NIR-I excitation about an order of magnitude brighter than from NIR-II excitation. The 55 μm spatial resolution achievable with this approach is demonstrated through imaging of aUCNPs in PDMS (polydimethylsiloxane) micro-wells, showing resolution of micrometer-scale targets with single-pixel precision. INSITE imaging of intratumoral NaEr0.8Yb0.2F4 aUCNPs shows a signal-to-background ratio of 9, limited only by photodiode dark current and electronic noise. Conclusion: This work demonstrates INSITE imaging of aUCNPs in tumors, achieving an imaging platform that is thinned to just a 25 μm-thin, planar form-factor, with both NIR-I and NIR-II excitation. Based on a highly paralleled array structure INSITE is scalable, enabling direct coupling with a wide array of surgical and robotic tools for seamless integration with tissue actuation, resection or ablation.
Keywords: NIR excitation; intraoperative imaging; time-resolved imaging; upconverting nanoparticle.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Karakiewicz PI, Eastham JA, Graefen M, Cagiannos I, Stricker PD, Klein E. et al. Prognostic impact of positive surgical margins in surgically treated prostate cancer: multi-institutional assessment of 5831 patients. Urology. 2005;66:1245–50. - PubMed
-
- Moran MS, Schnitt SJ, Giuliano AE, Harris JR, Khan SA, Horton J. et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. J Clin Oncol. 2014;32:1507–15. - PubMed
-
- Tendulkar RD, Agrawal S, Gao T, Efstathiou JA, Pisansky TM, Michalski JM. et al. Contemporary Update of a Multi-Institutional Predictive Nomogram for Salvage Radiotherapy After Radical Prostatectomy. J Clin Oncol. 2016;34:3648–54. - PubMed

