Chemical compound cinobufotalin potently induces FOXO1-stimulated cisplatin sensitivity by antagonizing its binding partner MYH9
- PMID: 31754475
- PMCID: PMC6861228
- DOI: 10.1038/s41392-019-0084-3
Chemical compound cinobufotalin potently induces FOXO1-stimulated cisplatin sensitivity by antagonizing its binding partner MYH9
Abstract
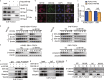
In this study, we present novel molecular mechanisms by which FOXO1 functions as a tumor suppressor to prevent the pathogenesis of nasopharyngeal carcinoma (NPC). First, we observed that FOXO1 not only controlled tumor stemness and metastasis, but also sensitized NPC cells to cisplatin (DDP) in vitro and in vivo. Mechanistic studies demonstrated that FOXO1-induced miR-200b expression through the GSK3β/β-catenin/TCF4 network-mediated stimulation of ZEB1, which reduced tumor stemness and the epithelial-mesenchymal transition (EMT) signal. Furthermore, we observed FOXO1 interaction with MYH9 and suppression of MYH9 expression by modulating the PI3K/AKT/c-Myc/P53/miR-133a-3p pathway. Decreased MYH9 expression not only reduced its interactions with GSK3β, but also attenuated TRAF6 expression, which then decreased the ubiquitin-mediated degradation of GSK3β protein. Increased GSK3β expression stimulated the β-catenin/TCF4/ZEB1/miR-200b network, which increased the downstream tumor stemness and EMT signals. Subsequently, we observed that chemically synthesized cinobufotalin (CB) strongly increased FOXO1-induced DDP chemosensitivity by reducing MYH9 expression, and the reduction in MYH9 modulated GSK3β/β-catenin and its downstream tumor stemness and EMT signal in NPC. In clinical samples, the combination of low FOXO1 expression and high MYH9 expression indicated the worst overall survival rates. Our studies demonstrated that CB potently induced FOXO1-mediated DDP sensitivity by antagonizing its binding partner MYH9 to modulate tumor stemness in NPC.
Keywords: Cancer genomics; Cancer stem cells; Head and neck cancer.
© The Author(s) 2019.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures





References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

