Treatment optimisation for hepatitis C in the era of combination direct-acting antiviral therapy: a systematic review and meta-analysis
- PMID: 31754636
- PMCID: PMC6854875
- DOI: 10.12688/wellcomeopenres.15411.1
Treatment optimisation for hepatitis C in the era of combination direct-acting antiviral therapy: a systematic review and meta-analysis
Abstract
Background: Prior to direct-acting antiviral (DAA) therapy, personalised medicine played an important role in the treatment of hepatitis C virus (HCV). Whilst simplified treatment strategies are central to treatment scale-up, some patients will benefit from treatment optimisation. This systematic review and meta-analysis explores treatment optimisation strategies in the DAA era. Methods: We systematically searched Medline, Embase, and Web of Science for studies that adopted a stratified or personalised strategy using a licensed combination DAA regimen, alone or with additional agents. We performed a thematic analysis to classify optimisation strategies and a meta-analysis of sustained virologic response rates (SVR), exploring heterogeneity with subgroup analyses and meta-regression. Results: We included 64 studies (9450 participants). Thematic analysis found evidence of three approaches: duration, combination, and/or dose optimisation. We separated strategies into those aiming to maintain SVR in the absence of predictors of failure, and those aiming to improve SVR in the presence of predictors of failure. Shortened duration regimens achieve pooled SVR rates of 94.2% (92.3-95.9%) for 8 weeks, 81.1% (75.1-86.6%) for 6 weeks, and 63.1% (39.9-83.7%) for ≤4 weeks. Personalised strategies (100% vs 87.6%; p<0.001) and therapy shortened according to ≥3 host/viral factors (92.9% vs 81.4% or 87.2% for 1 or 2 host/viral factors, respectively; p=0.008) offer higher SVR rates when shortening therapy. Hard-to-treat HCV genotype 3 patients suffer lower SVR rates despite treatment optimisation (92.6% vs 98.2%; p=0.001). Conclusions: Treatment optimisation for individuals with multiple predictors of treatment failure can offer high SVR rates. More evidence is needed to identify with confidence those individuals in whom SVR can be achieved with shortened duration treatment.
Keywords: direct acting antivirals; hepatitis C virus; personalised medicine; stratified medicine; sustained virologic response; systematic review; treatment optimisation.
Copyright: © 2019 Jones CR et al.
Conflict of interest statement
Competing interests: BFF has received a travel grant to attend a conference from Gilead Sciences. GSC has acted as an advisor and/or provided lectures for Gilead Sciences and Merck & Co. GSC is a steering group member for the STOP-HCV Consortium. GSC is the chief investigator for the STOP-HCV-1 trial.
Figures
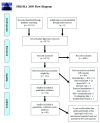

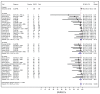
References
-
- WHO: Global Health Sector Strategy on Viral Hepatitis 2016-2021. Geneva, Switzerland,2016. Reference Source
Publication types
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

